Papers, communications and reviews… our recent published work is here.
Molecule/Semiconductor Hybrid Materials for Visible-Light CO2 Reduction: Design Principles and Interfacial Engineering
Acc. Mater. Res. 2 (6), 458–470, 2021
Because of increasing concerns over the depletion of energy sources and the concomitant increase in CO2 emissions, much attention has been devoted to carbon capture and utilization technologies. Among the various methods and schemes proposed, visible-light-driven CO2 reduction in combination with water oxidation, one of the representative models of artificial photosynthesis, is an attractive solution because it enables abundant water and inexhaustible solar energy to be used to produce value-added chemicals. Molecular metal complexes and semiconductors are promising candidates for photocatalysts that can reduce CO2 to CO, formate, formaldehyde, or other hydrocarbons. Although both molecular metal complexes and semiconductors have strengths and weaknesses, their weaknesses (low oxidation ability and low selectivity for reduction reactions) can be overcome via the construction of a suitable molecule/semiconductor hybrid material. However, facilitating electron transfer at the molecule/semiconductor junction while suppressing unfavorable back electron transfer events is challenging. Consequently, the number of molecule/semiconductor hybrid systems that show a reasonable level of visible-light photocatalytic activity is limited, despite the development of a large number of visible-light-driven semiconductors and molecular photocatalysts (or catalysts). In this Account, we describe our approaches to developing hybrid photocatalysts and photoelectrodes for CO2 reduction. We have been developing both molecular (photo)catalysts and semiconductor photocatalysts individually, the latter of which are also designed for visible-light water splitting. For example, supramolecular photocatalysts that possess both photosensitizer and catalyst units in a single molecule can reduce CO2 to formate or CO in a homogeneous system, with high selectivity toward the desired product and high quantum yields of several tens of percent. However, nonoxide semiconductors such as C/N-based polymers and mixed-anion compounds exhibit a strong photooxidation ability under visible light. Carefully designed molecule/semiconductor hybrid materials achieve CO2 reduction under visible light with high product selectivity and stability even in an aqueous environment, where the concentration of CO2 is low but that of protons is high. Visible-light CO2 reduction combined with H2O oxidation is possible via the construction of a photoelectrochemical cell that comprises a molecular photocathode and an n-type semiconductor photoanode. Although our photosystems can be regarded as model systems for artificial photosynthesis, their light-energy conversion efficiencies are still unsatisfactory. To improve the efficiency, materials design, including interfacial engineering at the molecule/semiconductor junction, is important and is the general theme of the results highlighted in this Account.
https://doi.org/10.1021/accountsmr.1c00060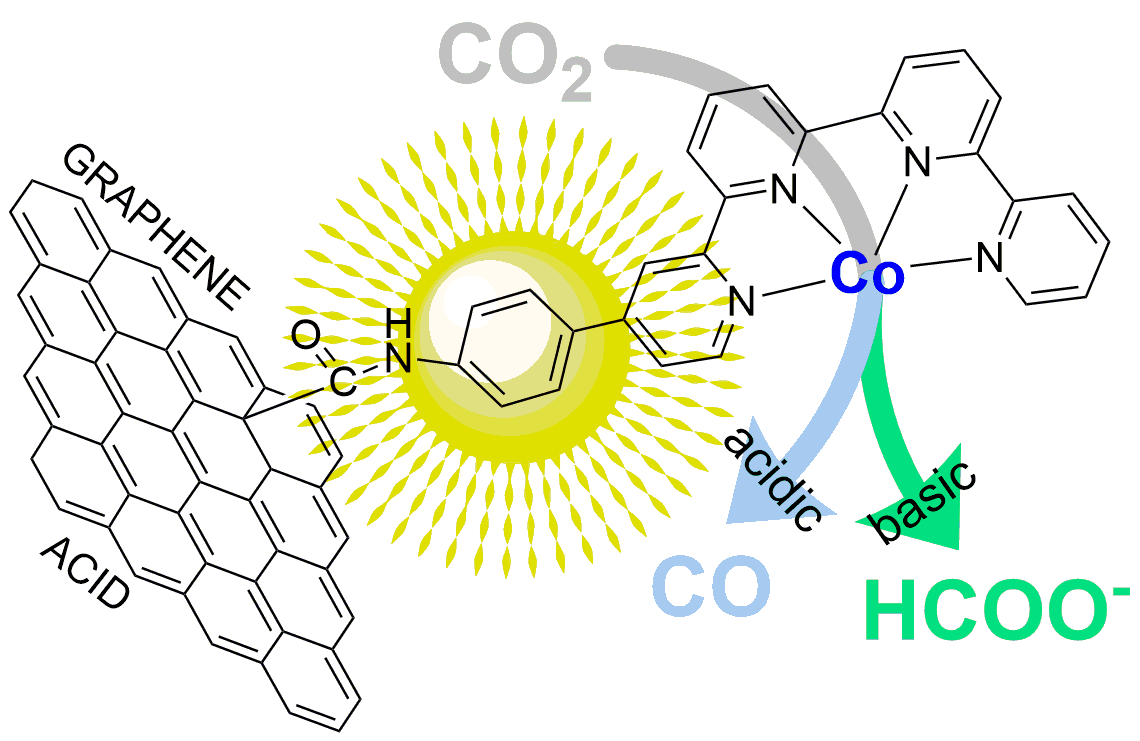
Hybridization of Molecular and Graphene Materials for CO2 Photocatalytic Reduction with Selectivity Control
J. Am. Chem. Soc. 143 (22), 8414-8425, 2021
In the quest for designing efficient and stable photocatalytic materials for CO2 reduction, hybridizing a selective noble-metal-free molecular catalyst and carbon-based light-absorbing materials has recently emerged as a fruitful approach. In this work, we report about Co quaterpyridine complexes covalently linked to graphene surfaces functionalized by carboxylic acid groups. The nanostructured materials were characterized by X-ray photoemission spectroscopy, X-ray absorption spectroscopy, IR and Raman spectroscopies, high-resolution transmission electron microscopy and proved to be highly active in the visible-light-driven CO2 catalytic conversion in acetonitrile solutions. Exceptional stabilities (over 200 h of irradiation) were obtained without compromising the selective conversion of CO2 to products (>97%). Most importantly, complete selectivity control could be obtained upon adjusting the experimental conditions: production of CO as the only product was achieved when using a weak acid (phenol or trifluoroethanol) as a co-substrate, while formate was exclusively obtained in solutions of mixed acetonitrile and triethanolamine.
https://doi.org/10.1021/jacs.1c02250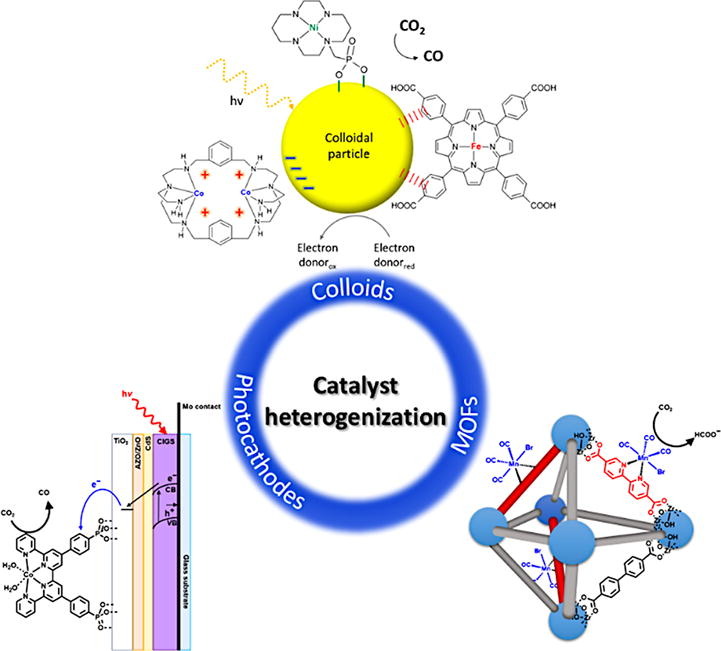
Light-Driven Catalytic Conversion of CO2 with Heterogenized Molecular Catalysts Based on Fourth Period Transition Metals
Coord. Chem. Rev. 443, article number 214018
This review examines recent advances in photocatalytic CO2 reduction using heterogenized molecular catalysts. The main part of the discussion is focused on the chemistry used to attach catalysts to different supports to produce hybrid materials, and how this effects photocatalytic performance. Examples of hybrid materials used for colloidal dispersions and solid suspensions are presented, including those based on carbon nitride, chalcogenide and perovskite quantum dots, and metal oxides. Some key examples in which this chemistry has been employed to make electrodes and photoelectrodes for photoelectrochemical CO2 reduction are also presented. In addition, the incorporation of molecular catalysts into ordered, porous frameworks (MOFs and COFs) is discussed because it offers many new and unique chemical pathways for heterogenization. Lastly, an outlook for this field and the potential future impact of these systems on solar fuels research is given.
https://doi.org/10.1016/j.ccr.2021.214018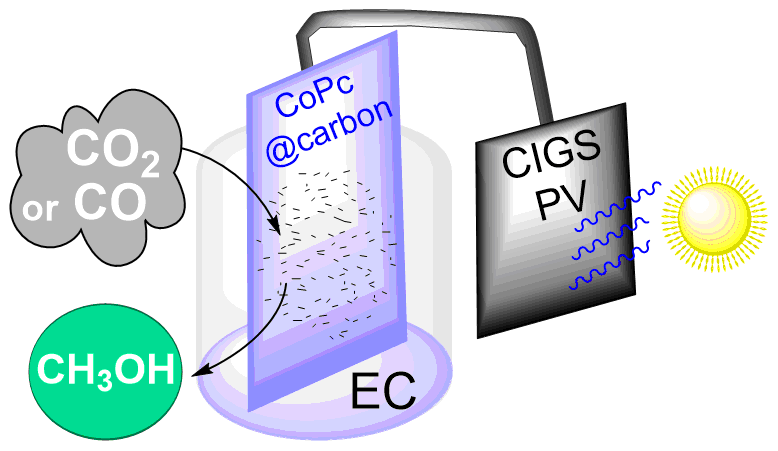
CO2 Reduction to Methanol with a Molecular Cobalt Catalyst Loaded Porous Carbon Electrode Assisted by a CIGS Photovoltaic Cell
ChemPhotoChem 5, 705-710, 2021
Conversion of CO2 into valuable compounds, including fuels, with renewable energy source and sustainable compounds is a challenge addressed by artificial photosynthesis research. In particular, solar assisted electrochemical (EC) processes, in which electrons are furnished by a photovoltaic (PV) cell is a promising approach. A PV‐EC system is described, consisting in a CIGS PV unit linked to a carbon electrode loaded with cobalt phthalocyanine as molecular catalyst, able to achieve the CO2 reduction to CO and then to methanol in aqueous media with limited bias voltage. Using CO as starting material, a partial current density of ca. 0.6 mA cm‐2 for methanol is obtained at a bias voltage corresponding to a low 240 mV overpotential. Remarkably, the liquid fuel production can be sustained for at least 7h. Under ideal conditions, the CO2‐to‐CH3OH reaction shows a global Faradaic efficiency of 28%.
https://doi.org/10.1002/cptc.202100035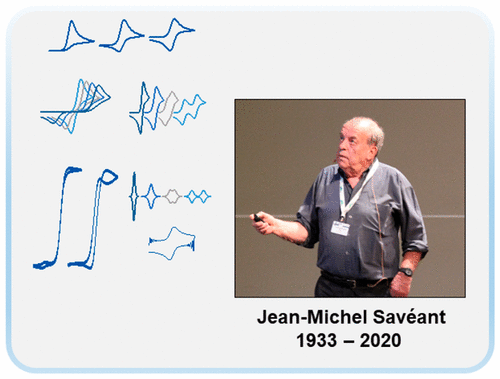
A Pioneering Career in Electrochemistry: Jean-Michel Savéant
ACS Catal. 11, 3224-3238, 2021
Prof. Jean-Michel Savéant sadly passed on August 16, 2020. We would like to honor his memory, his tremendous contribution to electrochemistry, and its use for a general understanding of the laws of physical chemistry. In this review, we highlight his decisive role in the foundation of molecular electrochemistry. We also present his major achievements in the field of molecular and biomolecular catalysis. Finally, we review his unique contribution to dissociative electron transfers and to the electrochemical approach of proton-coupled electron transfers. This shows how various concepts rigorously established and experimentally validated assemble to each other to enlighten complex systems.
https://doi.org/10.1021/acscatal.0c05632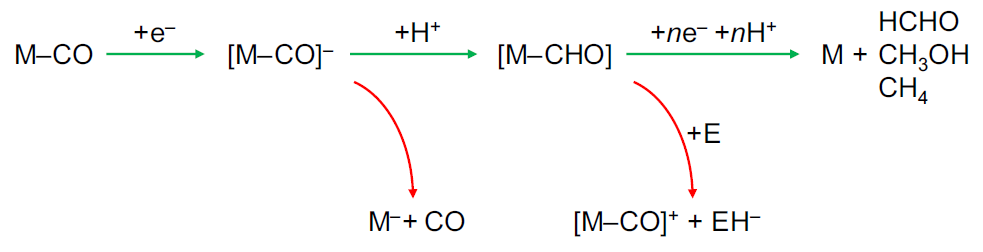
Molecular Electrochemical Reduction of CO2 beyond Two Electrons
Trends Chem. 3 (5), 359-372, 2021
CO2 molecular electrochemical reduction has recently led to remarkable advancements. High performing catalysts have been prepared that mainly produce carbon monoxide (CO) and formic acid (HCOOH). Studies reporting highly reduced products (formed with more than two electrons) remain scarce, but the field is now quickly emerging. Driving such multi-electron, multi-proton catalytic reactions with molecular catalysts would offer unlimited possibilities for tuning and controlling catalytic active site configuration/environment. In this perspective, we discuss known examples and draw a first unified reactivity scheme. Starting with reports on C1 products such as formaldehyde, methanol, and methane, we then address the formation of C–C bonds and associated intricate pathways. Eventually, we provide critical insights to sharpen the interpretation and analysis of existing and upcoming reports.
https://doi.org/10.1016/j.trechm.2021.02.003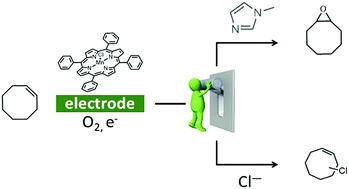
Modulating Alkene Reactivity from Oxygenation to Halogenation via Electrochemical O2 Activation by Mn Porphyrin
Chem. Commun. 57, 1198-1201, 2021
Oxidation of organic substrates is achieved in nature under mild conditions thanks to metalloenzymes but remains a challenge for chemists. Herein we show by UV-Vis spectroelectrochemistry that when MnIIITPPCl is electrochemically reduced to MnII in CH2Cl2 under O2, a MnIIO2˙ species is generated. Benzoic anhydride reacts with the latter triggering a catalytic current in cyclic voltammetry. Electrolysis on the catalytic wave in the presence of cyclooctene leads to its oxygenation or halogenation depending on the axial ligand present as reported here for the first time.
https://doi.org/10.1039/D0CC07531K
Carbon Dioxide Electrochemistry: Homogeneous and Heterogeneous Catalysis
Energy and Environment Series No. 28, The Royal Society of Chemistry
About the book Conversion of light and electricity to chemicals is an important component of a sustainable energy system. The exponential growth in renewable energy generation implies that there will be strong market pull for chemical energy storage technology in the near future, and here carbon dioxide utilization must play a central role. The electrochemical conversion of carbon dioxide is key in achieving these goals. Carbon Dioxide Electrochemistry showcases different advances in the field, and bridges the two worlds of homogeneous and heterogeneous catalysis that are often perceived as in competition in research. Chapters cover homogeneous and heterogeneous electrochemical reduction of CO2, nanostructures for CO2 reduction, hybrid systems for CO2 conversion, electrochemical reactors, theoretical approaches to catalytic reduction of CO2, and photoelectrodes for electrochemical conversion. With internationally well-known editors and authors, this book will appeal to graduate students and researchers in energy, catalysis, chemical engineering and chemistry who work on carbon dioxide. About Chapter 2 "Homogeneous Electrochemical Reduction of CO2. From Homogeneous to Supported Systems" The development of supported molecular catalysts deposited at conductive electrodes has recently (re)opened a wide range of new possibilities to investigate CO2 reduction with the catalytic selectivity traditionally offered by molecules, and the increased stability usually encountered with heterogeneous materials. The various methods for immobilizing molecules (non-covalent, covalent, periodic systems) are detailed as well as the performances of the most typical hybrid electrodes so far investigated. As in homogeneous conditions, electrochemical techniques provide the tools for thorough mechanistic studies. Models are described toward these ends and typical systems are analysed. Remarkably, these supported catalytic systems offer the most intriguing and stimulating avenues to bridge and possibly merge homogeneous and heterogeneous catalysis.
https://doi.org/10.1039/9781788015844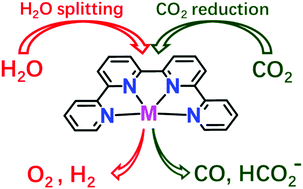
Molecular Quaterpyridine-based Metal Complexes for Small Molecule Activation: Water Splitting and CO2 Reduction
Chem. Soc. Rev. 49, 7271-7283, 2020
Artificial photosynthesis is considered as one of the most promising strategies for solar-to-fuel conversion through sunlight-driven water splitting and CO2 reduction. This tutorial describes recent developments in the use of metal quaterpyridine complexes as electrocatalyts and photocatalysts for artificial photosynthesis.
https://doi.org/10.1039/D0CS00927J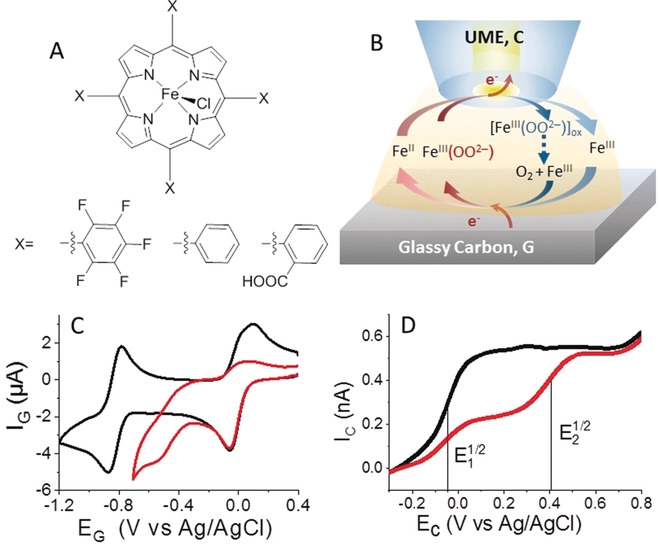
Probing the Activity of Iron Peroxo Porphyrin Intermediates in the Reaction Layer during the Electrochemical Reductive Activation of O2
Angew. Chem. Int. Ed. 59 (38), 16376-16380, 2020
Herein we report the first example of using scanning electrochemical microscopy (SECM) to quantitatively analyze O2 reductive activation in organic media catalyzed by three different Fe porphyrins. For each porphyrin, SECM can provide in one single experiment the redox potential of various intermediates, the association constant of FeII with O2, and the pKa of the FeIII(OOH-)/ FeIII(OO2-) couple. The results obtained can contribute to a further understanding of the parameters controlling the catalytic efficiency of the Fe porphyrin towards O2 activation and reduction.
https://doi.org/10.1002/anie.202004977