Papers, communications and reviews… our recent published work is here.
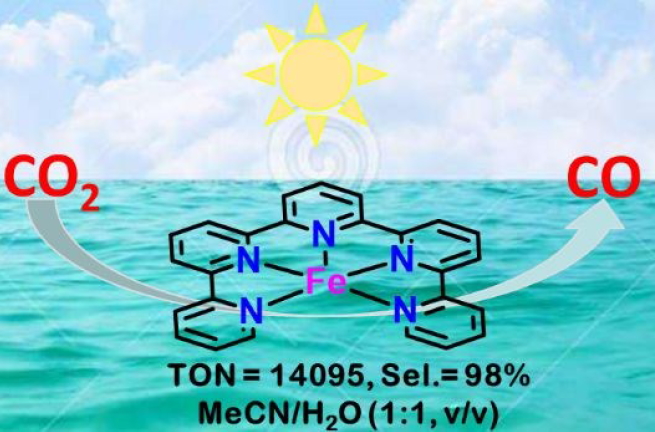
Highly Active and Robust Iron Quinquepyridine Complex for Photocatalytic CO2 Reduction in Aqueous Acetonitrile Solution
Chem. Commun. 56, 6249-6252, 2020
The iron(II) complex bearing the 2,2':6',2'':6'',2''':6''',2''''-quinquepyridine (qnpy) ligand, [Fe(qnpy)(OH2)2]2+, is a highly efficient and robust catalyst for photocatalytic reduction of CO2 to CO in aqueous acetonitrile. A turnover number (TON) for CO of up to 14095 with 98% selectivity can be achieved using Ru(phen)3Cl2 (phen = 1,10-phenanthroline) as photosensitizer and BIH (1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole) as sacrificial reductant in a CO2-saturated MeCN/H2O (1:1, v/v) solution under visible light irradiation. This Fe complex is state-of-the art for CO2 visible-light-driven catalysis.
https://doi.org/10.1039/D0CC01930E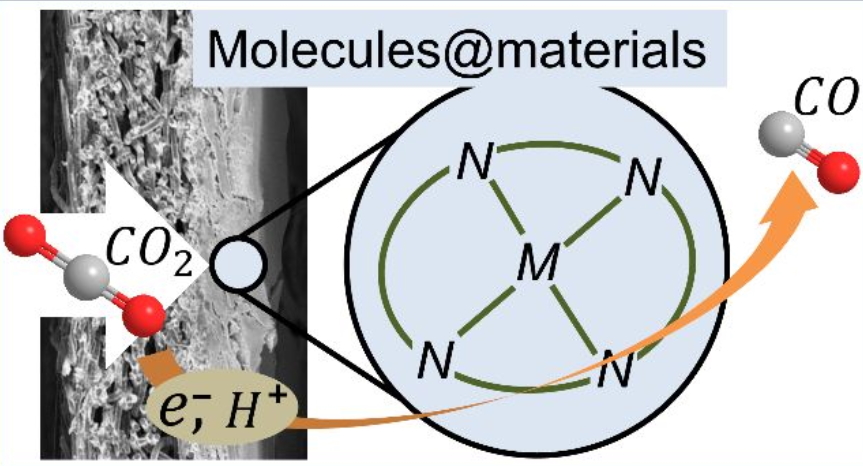
Molecular Catalysts Boost the Rate of Electrolytic CO2 Reduction
ACS Energy Lett. 5, 1512-1518, 2020
Electrolysis is a potential useful approach for converting carbon dioxide into chemicals and fuels. The most active electrocatalysts for efficiently mediating the carbon dioxide reduction reaction (CO2RR) have long been assumed to be solid silver, gold, or copper. However, there is an emerging body of data showing that molecular catalysts can operate at levels of performance commensurate with solid-state catalysts. These recent advances in deploying molecular catalysts present entirely new opportunities for understanding CO2RR in electrochemical reactors, and tailoring active sites for the selective formation of CO2RR products. This Perspective highlights the recent advances and the opportunities for the implementation of molecular electrocatalysts into carbon dioxide electrolyzers.
https://doi.org/10.1021/acsenergylett.0c00536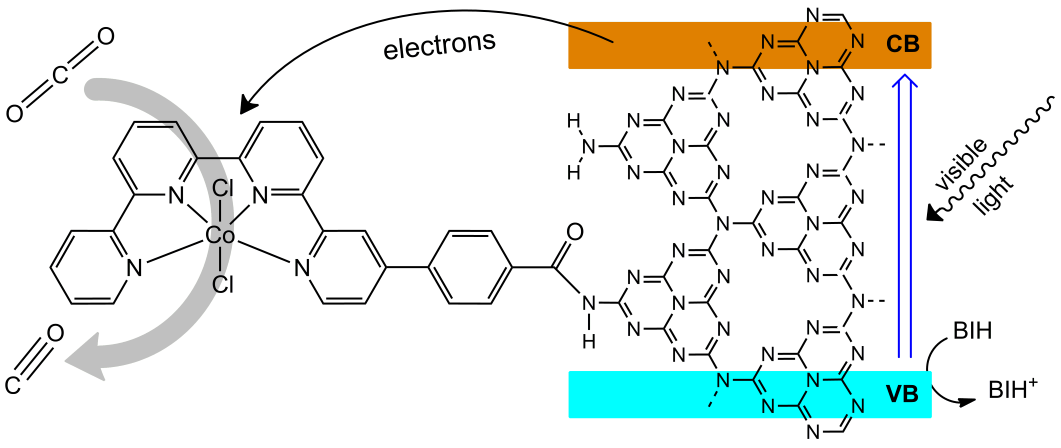
Efficient Visible-Light Driven CO2 Reduction by a Cobalt Molecular Catalyst Covalently Linked to Mesoporous Carbon Nitride
J. Am. Chem. Soc. 142 (13), 6188-6195, 2020
Achieving visible-light driven carbon dioxide reduction with high selectivity control and durability while using only earth abundant elements requires new strategies. Hybrid catalytic material was prepared upon covalent grafting a Co quaterpyridine molecular complex to semi-conductive mesoporous graphitic carbon nitride (mpg-C3N4) through an amide linkage. The molecular material was characterized by various spectroscopic techniques, including XPS, IR and impedance spectroscopy. It proved to be a selective catalyst for CO production in acetonitrile using a solar simulator with a high 98% selectivity, while being remarkably robust since no degradation was observed after 4 days of irradiation (ca. 500 catalytic cycles). This unique combination of a selective molecular catalyst with a simple and robust semi-conductive material opens new pathways for CO2 catalytic light-driven reduction.
https://doi.org/10.1021/jacs.9b13930
Manifesto for the routine use of NMR for the liquid product analysis of aqueous CO2 reduction: from comprehensive chemical shift data to formaldehyde quantification in water
Dalton Trans. 49, 4257-4265, 2020
CO2 reduction research is at a critical turnaround since it has the potential to partially or even substantially fulfil future clean energy needs. CO2-to-CO electrochemical conversion is getting closer from industrial implementation requirements. Efforts are now more and more directed to obtain highly reduced products such as methanol, methane, ethylene, ethanol, etc., most of them being liquids. Gas-phase products (e.g., CO, CH4) are typically detected and quantified by well-defined gas chromatography (GC and GC/MS) protocols. On the other hand, NMR, GC-MS, HPLC have been used for the liquid phase characterization, but no routine technique has yet been established, mainly due to lack of versatility of a single technique. Additionally, except NMR and GC-MS, classical techniques cannot distinguish 13C from 12C products, although it is a mandatory step to assess products origin. Herein, we show the efficiency and applicability of 1H NMR as routine technique for liquid phase products analysis and we address two previous shortcomings. We first established a comprehensive 1H and 13C NMR chemical shifts list for all 12CO2 and 13CO2 reduction products in water ranging from C1 to C3. Then we overcame the difficulty of identifying aqueous formaldehyde intermediate by 1H NMR through an efficient chemical trapping step, along with isotopic signature study. Formaldehyde can be reliably quantified in water with a concentration as low as 50 μM.
https://doi.org/10.1039/C9DT04749B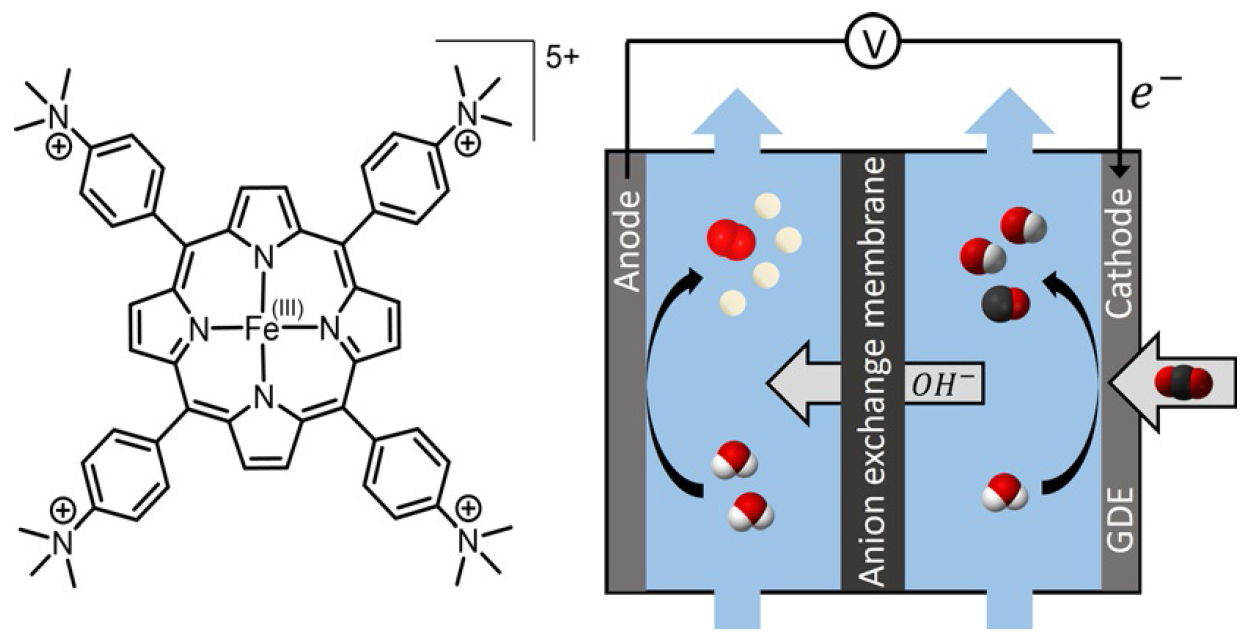
Iron Porphyrin Allows Fast and Selective Electrocatalytic Conversion of CO2 to CO in a Flow Cell
Chem. Eur. J. 26 (14), 3034-3038, 2020
Molecular catalysts have been shown to have high selectivity for CO2 electrochemical reduction to CO, but with current densities significantly below those obtained with solid‐state materials. By depositing a simple Fe porphyrin mixed with carbon black onto a carbon paper support, it was possible to obtain a catalytic material that could be used in a flow cell for fast and selective conversion of CO2 to CO. At neutral pH (7.3) a current density as high as 83.7 mA cm−2 was obtained with a CO selectivity close to 98 %. In basic solution (pH 14), a current density of 27 mA cm−2 was maintained for 24 h with 99.7 % selectivity for CO at only 50 mV overpotential, leading to a record energy efficiency of 71 %. In addition, a current density for CO production as high as 152 mA cm−2 (>98 % selectivity) was obtained at a low overpotential of 470 mV, outperforming state‐of‐the‐art noble metal based catalysts.
https://doi.org/10.1002/chem.202000160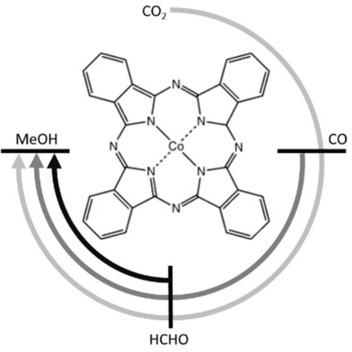
Aqueous Electrochemical Reduction of Carbon Dioxide and Carbon Monoxide into Methanol with Cobalt Phthalocyanine
Angew. Chem. Int. Ed. 58 (45), 16172-16176, 2019
Conversion of CO2 into valuable molecules is a field of intensive investigation with the aim of developing scalable technologies for making fuels using renewable energy sources. While electrochemical reduction into CO and formate are approaching industrial maturity, a current challenge is obtaining more reduced products like methanol. However, literature on the matter is scarce, and even more for the use of molecular catalysts. Here, we demonstrate that cobalt phthalocyanine, a well‐known catalyst for the electrochemical conversion of CO2 to CO, can also catalyze the reaction from CO2 or CO to methanol in aqueous electrolytes at ambient conditions of temperature and pressure. The studies identify formaldehyde as a key intermediate and an unexpected pH effect on selectivity. This paves the way for establishing a sequential process where CO2 is first converted to CO which is subsequently used as a reactant to produce methanol. Under ideal conditions, the reaction shows a global Faradaic efficiency of 19.5 % and chemical selectivity of 7.5 %.
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201909257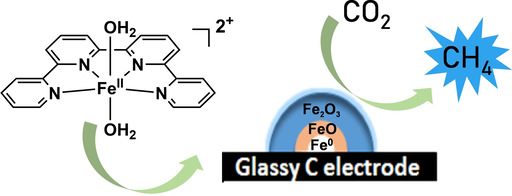
An Iron Quaterpyridine Complex as Precursor for the Electrocatalytic Reduction of CO2 to Methane
ChemSusChem 12 (19), 4500-4505, 2019
A Fe quaterpyridine complex was used as a molecular precursor for the electrochemical reduction of CO2 to CH4 in acetonitrile in the presence of triethanolamine. CH4 was produced with a faradaic yield of approximately 2.1 % at 25 °C and 1 atm pressure of CO2 as reactant. Controlled potential electrolysis coupled to ex situ X‐ray photoelectron spectroscopy and X‐ray absorption spectroscopy of the electrode surface revealed the formation of metallic iron covered by iron oxides as species responsible for catalysis.
https://onlinelibrary.wiley.com/doi/abs/10.1002/cssc.201902040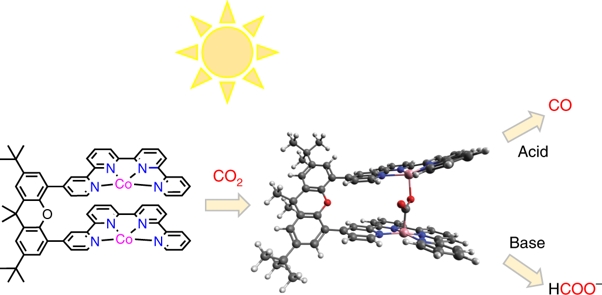
Selectivity control of CO versus formate production in the visible-light-driven catalytic reduction of CO2 with two cooperative metal sites
Nature Catal. 2 (9), 801-808, 2019
It is highly desirable to discover molecular catalysts with controlled selectivity for visible-light-driven CO 2 reduction to fuels. In the design of catalysts employing earth-abundant metals, progress has been made for CO production, but formate generation has been observed more rarely. Here, we report a binuclear Co complex bearing a bi-quaterpyridine ligand that can selectively reduce CO 2 to HCOO− or CO under visible light irradiation. Selective formate production (maximum of 97%) was obtained with a turnover number of up to 821 in basic acetonitrile solution. Conversely, in the presence of a weak acid, CO 2 reduction affords CO with high selectivity (maximum of 99%) and a maximum turnover number of 829. The catalytic process is controlled by the two Co atoms acting synergistically, and the selectivity can be steered towards the desired product by simply changing the acid co-substrate.
https://www.nature.com/articles/s41929-019-0331-6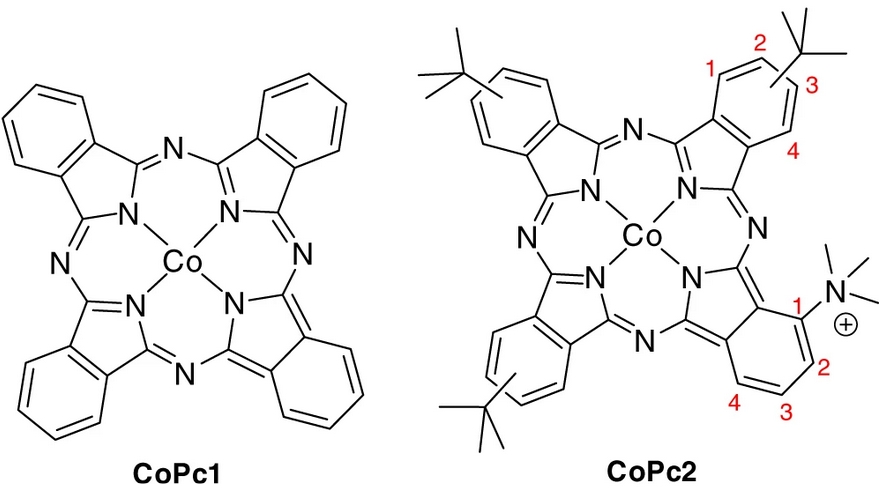
CO2 Electrochemical Catalytic Reduction with a Highly Active Cobalt Phthalocyanine
Nature Commun. 10 (1), 1-8, 2019
Molecular catalysts that combine high product selectivity and high current density for CO2 electrochemical reduction to CO or other chemical feedstocks are urgently needed. While earth-abundant metal-based molecular electrocatalysts with high selectivity for CO2 to CO conversion are known, they are characterized by current densities that are significantly lower than those obtained with solid-state metal materials. Here, we report that a cobalt phthalocyanine bearing a trimethyl ammonium group appended to the phthalocyanine macrocycle is capable of reducing CO2 to CO in water with high activity over a broad pH range from 4 to 14. In a flow cell configuration operating in basic conditions, CO production occurs with excellent selectivity (ca. 95%), and good stability with a maximum partial current density of 165 mA cm−2 (at −0.92 V vs. RHE), matching the most active noble metal-based nanocatalysts. These results represent state-of-the-art performance for electrolytic carbon dioxide reduction by a molecular catalyst.
https://www.nature.com/articles/s41467-019-11542-w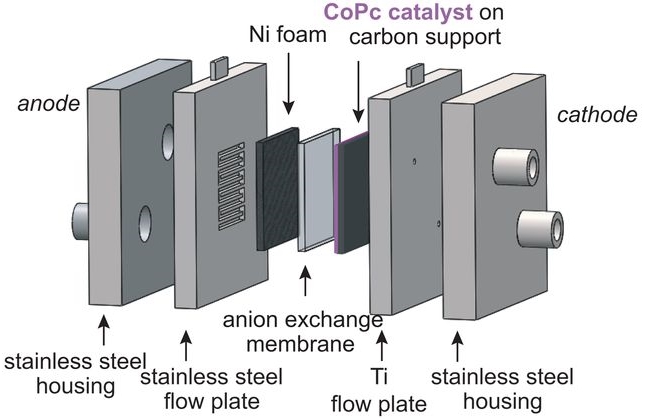
Molecular Electrocatalysts Can Mediate Fast, Selective CO2 Reduction in a Flow Cell
Science 365 (6451), 367-369, 2019
Practical electrochemical carbon dioxide (CO2) conversion requires a catalyst capable of mediating the efficient formation of a single product with high selectivity at high current densities. Solid-state electrocatalysts achieve the CO2 reduction reaction (CO2RR) at current densities ≥ 150 milliamperes per square centimeter (mA/cm2), but maintaining high selectivities at high current densities and efficiencies remains a challenge. Molecular CO2RR catalysts can be designed to achieve high selectivities and low overpotentials but only at current densities irrelevant to commercial operation. We show here that cobalt phthalocyanine, a widely available molecular catalyst, can mediate CO2 to CO formation in a zero-gap membrane flow reactor with selectivities > 95% at 150 mA/cm2. The revelation that molecular catalysts can work efficiently under these operating conditions illuminates a distinct approach for optimizing CO2RR catalysts and electrolyzers.
https://science.sciencemag.org/content/365/6451/367.abstract