Papers, communications and reviews… our recent published work is here.
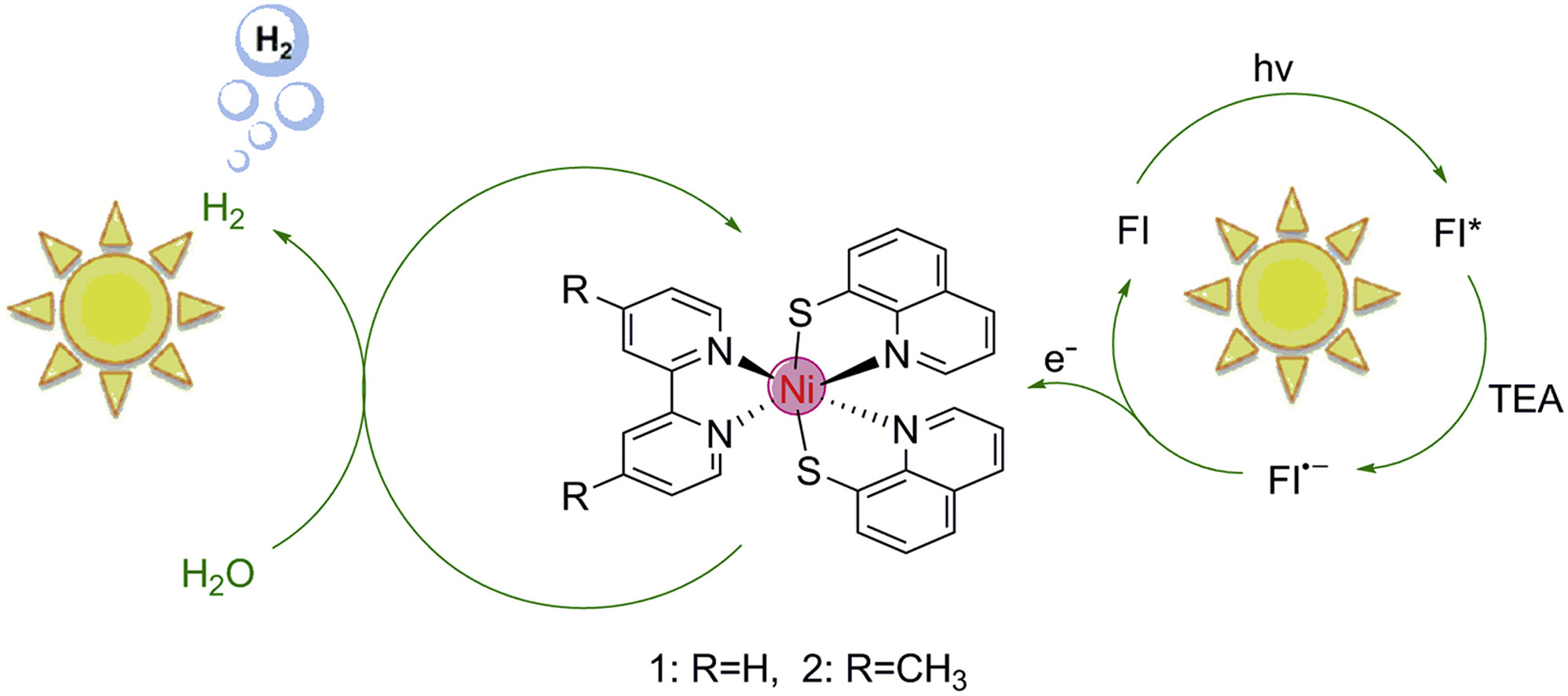
Highly Efficient Photocatalytic Hydrogen Evolution from Nickel Quinolinethiolate Complexes under Visible Light Irradiation
J. Power Sources 324, 253-260, 2016
Earth-abundant metal complexes have emerged as promising surrogates of platinum for catalyzing the hydrogen evolution reaction (HER). In this study, we report the design and synthesis of two novel nickel quinolinethiolate complexes, namely [Ni(Hqt)2(4, 4′-Z-2, 2′-bpy)] (Hqt = 8-quinolinethiol, Z = H [1] or CH3 [2], bpy = bipyridine). An efficient three-component photocatalytic homogeneous system for hydrogen generation working under visible light irradiation was constructed by using the target complexes as catalysts, triethylamine (TEA) as sacrificial electron donor and xanthene dyes as photosensitizer. We obtain turnover numbers (TON, vs. catalyst) for H2 evolution of 5923/7634 under the optimal conditions with 5.0 × 10−6 M complex 1/2 respectively, 1.0 × 10−3 M fluorescein and 5% (v/v) TEA at pH 12.3 in EtOH/H2O (1:1, v/v) mixture after 8 h irradiation (λ > 420 nm). We discuss the mechanism of H2 evolution in the homogeneous photocatalytic system based on fluorescence spectrum and cyclic voltammetry data.
https://doi.org/10.1016/j.jpowsour.2016.05.095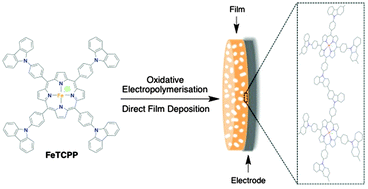
Controlled Electropolymerisation of a Carbazole-Functionalised Iron Porphyrin Electrocatalyst for CO2 Reduction
Chemical Communications 52 (34), 5864-5867, 2016
Using a one-step electropolymerisation procedure, CO2 absorbing microporous carbazole-functionalised films of iron porphyrins are prepared in a controlled manner. The electrocatalytic reduction of CO2 for these films is investigated to elucidate their efficiency and the origin of their ultimate degradation.
https://doi.org/10.1039/c6cc00982d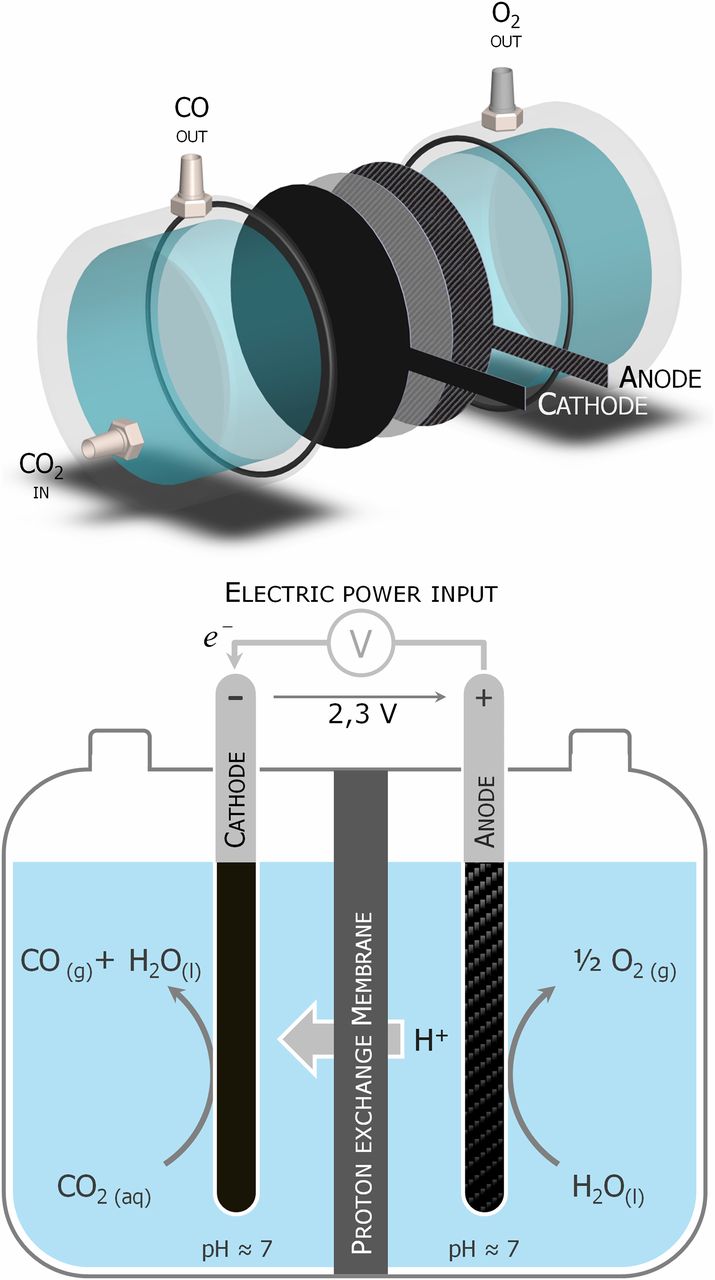
Efficient Electrolyzer for CO2 Splitting in Neutral Water using Earth-Abundant Materials
Proc. Natl. Acad. Sci. U.S.A. 113 (20), 5526-5529, 2016
Low-cost, efficient CO2-to-CO+O2 electrochemical splitting is a key step for liquid-fuel production for renewable energy storage and use of CO2 as a feedstock for chemicals. Heterogeneous catalysts for cathodic CO2-to-CO associated with an O2-evolving anodic reaction in high-energy-efficiency cells are not yet available. An iron porphyrin immobilized into a conductive Nafion/carbon powder layer is a stable cathode producing CO in pH neutral water with 90% faradaic efficiency. It is coupled with a water oxidation phosphate cobalt oxide anode in a home-made electrolyzer by means of a Nafion membrane. Current densities of approximately 1 mA/cm2 over 30-h electrolysis are achieved at a 2.5-V cell voltage, splitting COsub>2 and H2O into CO and O2 with a 50% energy efficiency. Remarkably, CO2 reduction outweighs the concurrent water reduction. The setup does not prevent high-efficiency proton transport through the Nafion membrane separator: The ohmic drop loss is only 0.1 V and the pH remains stable. These results demonstrate the possibility to set up an efficient, low-voltage, electrochemical cell that converts CO2 into CO and O2 by associating a cathodic-supported molecular catalyst based on an abundant transition metal with a cheap, easy-to-prepare anodic catalyst oxidizing water into O2.
https://doi.org/10.1073/pnas.1604628113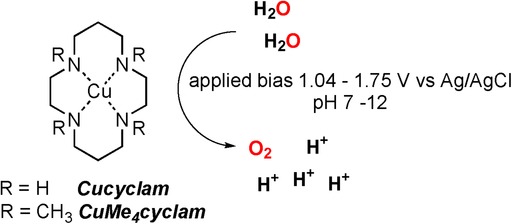
Heterogeneous and Homogeneous Routes in Water Oxidation Catalysis Starting from Cu(II) Complexes with Tetraaza Macrocyclic Ligands
Chem. As. J. 11 (8), 1281-1287, 2016
Since the first report in 2012, molecular copper complexes have been proposed as efficient electrocatalysts for water oxidation reactions, carried out in alkaline/neutral aqueous media. However, in some cases the copper species have been recognized as precursors of an active copper oxide layer, electrodeposited onto the working electrode. Therefore, the question whether copper catalysis is molecular or not is particularly relevant in the field of water oxidation. In this study, we investigate the electrochemical activity of copper(II) complexes with two tetraaza macrocyclic ligands, distinguishing heterogeneous or homogeneous processes depending on the reaction media. In an alkaline aqueous solution, and upon application of an anodic bias to working electrodes, an active copper oxide layer is observed to electrodeposit at the electrode surface. Conversely, water oxidation in neutral aqueous buffers is not associated to formation of the copper oxide layer, and could be exploited to evaluate and optimize a molecular, homogeneous catalysis.
https://doi.org/10.1002/asia.201501446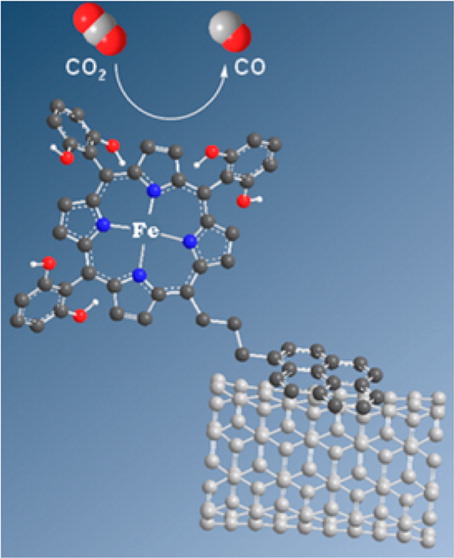
Noncovalent Immobilization of a Molecular Iron-based Electrocatalyst on Carbon Electrodes for Selective, Efficient CO2-to-CO Conversion in Water
J. Am. Chem. Soc. 138 (8), 2492-2495, 2016
Catalysis of fuel-producing reactions can be transferred from homogeneous solution to surface via attachment of the molecular catalyst. A pyrene-appended iron triphenyl porphyrin bearing six pendant OH groups on the phenyl rings in all ortho and ortho′ positions was immobilized on carbon nanotubes via noncovalent interactions and further deposited on glassy carbon. X-ray photoelectron spectroscopy and electrochemistry confirm catalyst immobilization. Using the carbon material, highly selective and rapid catalysis of the reduction of CO2 into CO occurs in water (pH 7.3) with 480 mV overpotential. Catalysis could be sustained for hours without loss of activity and selectivity, and high turnover number was obtained.
https://doi.org/10.1021/jacs.5b12652