Papers, communications and reviews… our recent published work is here.
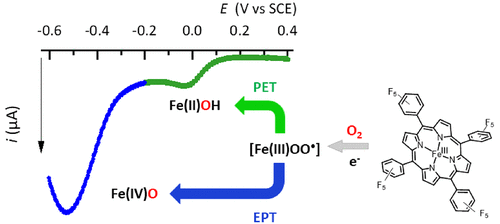
Electrocatalytic O2 Activation by Fe Tetrakis(pentafluorophenyl)porphyrin in Acidic Organic Media. Evidence of High-Valent Fe Oxo Species
Inorg. Chem. 59 (16), 11577–11583, 2020
O2 activation under mild conditions remains a weighty challenge for chemists. Herein we report a study of electrochemical O2 reductive activation catalyzed by FeIII(F20TPP)Cl, by means of cyclic voltammetry and UV–vis spectroelectrochemistry in acidic solutions of N,N-dimethylformamide. Two parallel catalytic pathways have been evidenced occurring at different overpotentials. At high overpotential a classical electron–proton (EPT) pathway where protonation of Fe peroxo ultimately leads to the formation of high-valent Fe oxo species dominates. At low overpotential a proton–electron (PET) pathway involving a hydrosuperoxo species has been identified.
https://doi.org/10.1021/acs.inorgchem.0c01379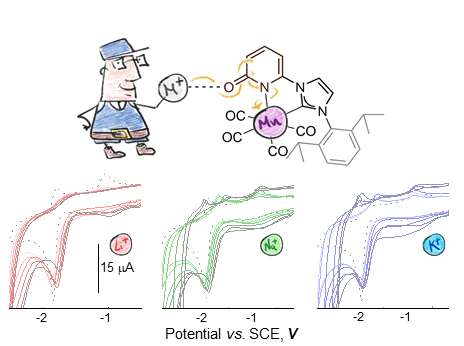
Mn(I) Complex Redox Potential Tunability by Remote Lewis Acid Interaction
Dalton Trans. 49, 16623-16626, 2020
In this work we report the synthesis and characterization of the coordinatively saturated MnI‒complex [Mn(L1)(CO)4] (1). Where L1 is a newly synthesized N-heterocyclic carbene (NHC) ligand containing a 2‒pyridone ring that holds an O‒atom directed at the second coordination sphere of the metal center. NMR spectroscopic studies unequivocally demonstrate the weak interactions of the [M]+ with 1, through the O‒atom from the 2‒pyridone fragment in L1, in DMF-d7 solutions. Additionally, we have successfully confirmed and described by electrochemical means the effects from these interactions on the electronic properties at the MnI‒center. Including the 1:1 ratio interaction between the [M]+ and complex 1 during the electron transfer. Finally, we evaluate the impact that the different alkali cations have on the electrochemical performance of the Mn‒complex under carbon dioxide. We demonstrate that electronic tuning at the metal center can be achieved through the remote interactions of alkali cations using the synthesized N-heterocyclic carbene ligand, containing the 2‒pyridone fragment. Broadening the potential of 2‒pyridone-based ligands from proton responsive platforms to redox tuner in the presence of alkali cations.
https://doi.org/10.1039/D0DT02467H
Hydrogenative Depolymerization of Nylons
J. Am. Chem. Soc. 142 (33), 14267–14275, 2020
The widespread crisis of plastic pollution demands discovery of new and sustainable approaches to degrade robust plastics such as nylons. Using a green and sustainable approach based on hydrogenation, in the presence of a ruthenium pincer catalyst at 150 °C and 70 bar H2, we report here the first example of hydrogenative depolymerization of conventional, widely used nylons and polyamides, in general. Under the same catalytic conditions, we also demonstrate the hydrogenation of a polyurethane to produce diol, diamine, and methanol. Additionally, we demonstrate an example where monomers (and oligomers) obtained from the hydrogenation process can be dehydrogenated back to a poly(oligo)amide of approximately similar molecular weight, thus completing a closed loop cycle for recycling of polyamides. Based on the experimental and density functional theory studies, we propose a catalytic cycle for the process that is facilitated by metal–ligand cooperativity. Overall, this unprecedented transformation, albeit at the proof of concept level, offers a new approach toward a cleaner route to recycling nylons.
https://doi.org/10.1021/jacs.0c05675
Molecular Catalysis of CO2 Reduction: Recent Advances and Perspectives in Electrochemical and Light-Driven Processes with Selected Fe, Ni and Co Aza Macrocyclic and Polypyridine Complexes
Chem. Soc. Rev. 49, 5772-5809, 2020
Earth-abundant Fe, Ni, and Co aza macrocyclic and polypyridine complexes have been thoroughly investigated for CO2 electrochemical and visible-light-driven reduction. Since the first reports in the 1970s, an enormous body of work has been accumulated regarding the two-electron two-proton reduction of the gas, along with mechanistic and spectroscopic efforts to rationalize the reactivity and establish guidelines for structure–reactivity relationships. The ability to fine tune the ligand structure and the almost unlimited possibilities of designing new complexes have led to highly selective and efficient catalysts. Recent efforts toward developing hybrid systems upon combining molecular catalysts with conductive or semi-conductive materials have converged to high catalytic performances in water solutions, to the inclusion of these catalysts into CO2 electrolyzers and photo-electrochemical devices, and to the discovery of catalytic pathways beyond two electrons. Combined with the continuous mechanistic efforts and new developments for in situ and in operando spectroscopic studies, molecular catalysis of CO2 reduction remains a highly creative approach.
https://doi.org/10.1039/D0CS00218F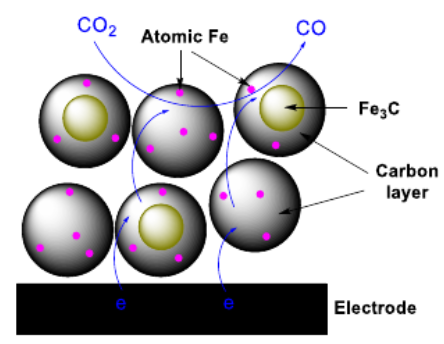
Achieving Near‐Unity CO Selectivity for CO2 Electroreduction on an Iron‐Decorated Carbon Material
ChemSusChem, 13, 6360-6369, 2020
We report a straightforward procedure to prepare a porous carbon material decorated with iron by direct pyrolysis of a mixture of a porous polymer and iron chloride. Characterization of the material with X‐ray diffraction, X‐ray absorption spectroscopy and electron microscopy points to the existence of iron carbide nanoparticles encapsulated inside the carbon matrix, while the elemental mapping and cyanide poisoning experiments demonstrate the presence of atomic Fe centers, albeit of trace amount, which are active sites for electrochemical CO2 reduction. The encapsulated iron carbide nanoparticles are found to boost the catalytic activity of atomic Fe sites existing in the outer carbon layer, rendering the material highly active and selective for CO2 reduction, although these atomic Fe sites are only present in trace amount. The target material exhibits a near‑unity selectivity (98%) for CO2‐to‐CO conversion at a small overpotential (410 mV) in water. Furthermore, the material holds potential for applications, as a current density over 30 mA cm-2 and a selectivity of 93% can be achieved in a flow cell.
https://doi.org/10.1002/cssc.202001311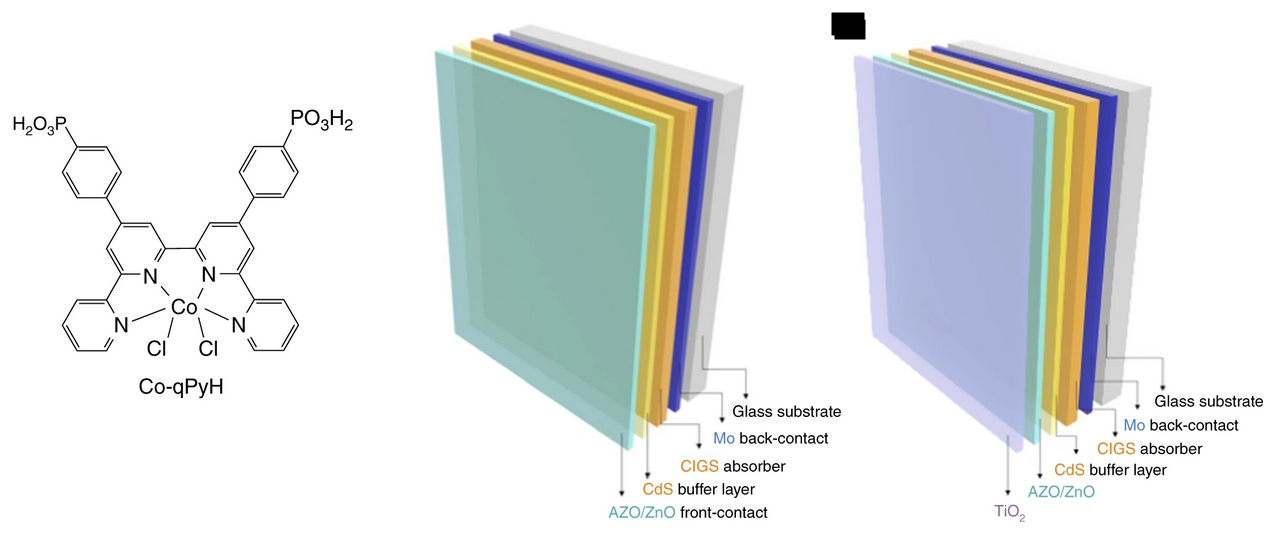
Photocathode Functionalized with a Molecular Cobalt Catalyst for Selective Carbon Dioxide Reduction in Water
Nature Commun. 11, 3499, 2020
Artificial photosynthesis is a vibrant field of research aiming at converting abundant, low energy molecules such as water, nitrogen or carbon dioxide into fuels or useful chemicals by means of solar energy input. Photo-electrochemical reduction of carbon dioxide is an appealing strategy, aiming at reducing the greenhouse gas into valuable products such as carbon monoxide at low or without bias voltage. Yet, in such configuration, there is no catalytic system able to produce carbon monoxide selectively in aqueous media with high activity, and using earth-abundant molecular catalyst. Upon associating a p-type Cu(In,Ga)Se2 semi-conductor with cobalt quaterpyridine complex, we herein report a photocathode complying with the aforementioned requirements. Pure carbon dioxide dissolved in aqueous solution (pH 6.8) is converted to carbon monoxide under visible light illumination with partial current density above 3 mA cm-2 and 97% selectivity, showing good stability over time.
https://doi.org/10.1038/s41467-020-17125-4
Electrochemical Conversion of CO2 to CO by a Competent Fe(I) Intermediate Bearing a Schiff Base Ligand
ChemSusChem 13 (16), 4111-4116, 2020
Iron complexes with a N2O2 type, N,N′‐o‐phenylenebis(salicylimine) salophen ligand, catalyze the electrochemical reduction of CO2 to CO in acetonitrile with phenol as the proton donor leading to 90 ÷ 99% selectivity, Faradaic efficiency up to 58%, and turnover frequency up to 103 s–1 at an overpotential of 0.65 V. This novel class of molecular catalyst for CO2 reduction operate through a mononuclear Fe I intermediate, with phenol being involved in the process with first order kinetics. The molecular nature of the catalyst and the low cost, easy synthesis and functionalization of the salophen ligand paves the way for catalyst engineering and optimization. Competitive electrodeposition of the coordination complex at the electrode surface results in the formation of iron based nanoparticles, which are active towards heterogeneous electrocatalytic processes mainly leading to proton reduction to hydrogen (Faradaic efficiency up to 80%), but also to the direct reduction of CO2 to methane with a Faradaic efficiency of 1 ÷ 2%.
https://doi.org/10.1002/cssc.202001143
Emergence of CO2 Electrolyzers Including Supported Molecular Catalysts
Curr. Opin. Electrochem. 24, 49-55, 2020
With access to cheap, sustainable electricity, electrocatalysis is a promising technology for converting electric power into storable chemical fuels or value added chemical compounds. This has sparked the development of electrocatalysts that need to operate at high product selectivity and high energy efficiency. Electrocatalytic alcohol oxidation, oxygen activation, nitrogen and carbon dioxide reduction are examples of reactions with a huge industrial potential. Notably, electrocatalytic reduction of carbon dioxide has recently developed as a favourable pathway to convert this greenhouse gas into value added chemicals and fuels. Earth abundant metals stabilized by carbon/nitrogen macrocycle ligands are well-known efficient and selective catalysts for the electrochemical reduction of CO2 in homogeneous conditions. Recently, such catalysts have also been used in supported conditions and implemented in flow cell electrolyzers, showing promising performances. This review provides a synopsis for the evolution of CO2 electrolyzers employing molecular catalysts.
https://doi.org/10.1016/j.coelec.2020.07.001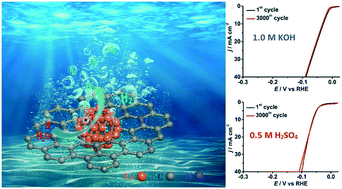
pH Universal Ru@N-doped Carbon Catalyst for Efficient and Fast Hydrogen Evolution
Catal. Sci. Technol. 10, 4405-4411, 2020
The development of efficient and cost-effective electrocatalysts for the hydrogen evolution reaction (HER) is of intense interest because H2 is one of the most promising renewable energy sources. Herein, we report a highly efficient and stable HER electrocatalyst composed of ruthenium nanoparticles embedded in nitrogen-doped carbon (NC), which is synthesized via a simple thermolysis process using a ruthenium complex as metal precusor and using ethylenediaminetetraacetic acid tetrasodium (Na4EDTA) salt as ligand and carbon source. It is found that the amount of Na4EDTA employed plays an important role in achieving sutiable and uniform Ru nanoparticles. The resulting Ru@NC(1:5) was found to exhibit excellent HER activity and robust stability in alkaline media (1.0 M KOH) with a low overpotential at 10 mA cm−2 (29 mV), small Tafel slope (27 mV per decade) and a high turnover frequency (TOF) of 0.96 s−1 at an overpotential of 50 mV, which are comparable to the state-of-the-art commercial Pt/C catalyst. Based on the characterization of the samples and the electrochemical measurements, this high performance of Ru@NC(1:5) is ascribed to its smallest particle size (ca. 2.1 nm diameter), large active site density and the high electrochemical conductivity by the N-doped carbon support. In addition, Ru@NC(1:5) also works well in acidic media (0.5 M H2SO4) indicating it is a pH-universal catalyst.
https://doi.org/10.1039/C9CY02552A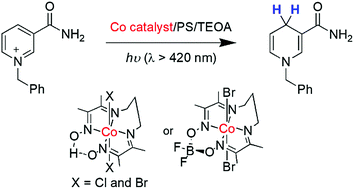
Precious-metal free Photocatalytic Production of a NADH Analogue using Cobalt Diimine-dioxime Catalysts in both Aqueous and Organic Conditions
Chem. Commun. 56, 7491-7494, 2020
The photocatalytic generation of a NADH synthetic analogue, i.e. 1-benzyl-1,4-dihydronicotinamide (1,4-BNAH) has been studied using cobalt diimino-dioxime complexes and the BF2-bridged derivative as catalysts. 1,4-BNAH was produced in both aqueous and organic media at unprecedented turnover numbers with metal and organic photosensitizers respectively.
https://doi.org/10.1039/D0CC02604B