Papers, communications and reviews… our recent published work is here.
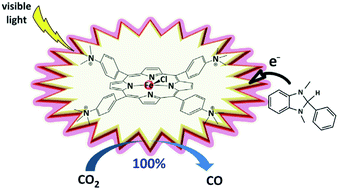
Non-sensitized Selective Photochemical Reduction of CO2 to CO under Visible Light with an Iron Molecular Catalyst
Chem. Commun. 53 (19), 2830-2833, 2017
A substituted tetraphenyl iron porphyrin, bearing positively charged trimethylammonio groups at the para position of each phenyl ring, demonstrates its ability as a homogeneous molecular catalyst to selectively reduce CO2 to CO under visible light irradiation in organic media without the assistance of a sensitizer and no competitive hydrogen evolution for several days.
https://doi.org/10.1039/c6cc09967j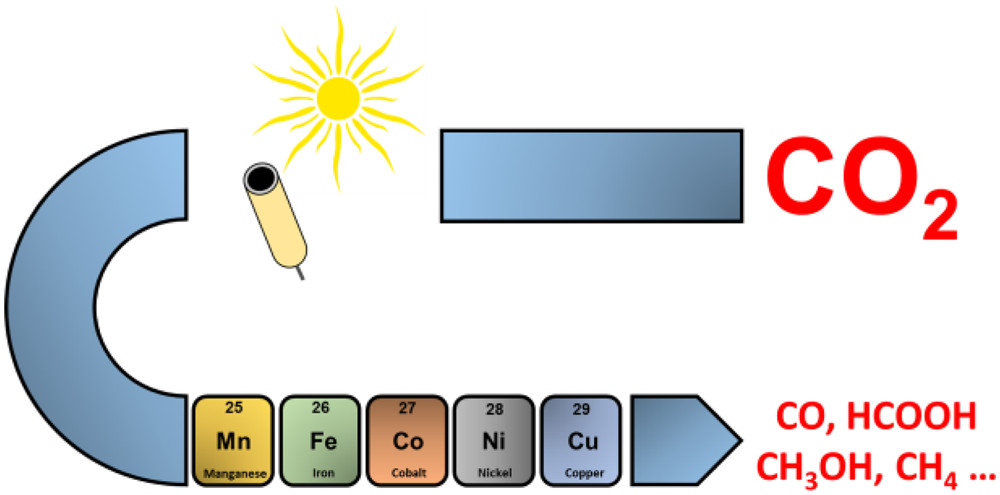
Electrons, Photons, Protons and Earth Abundant Metal Complexes for Molecular Catalysis of CO2 Reduction
ACS Catal. 7 (1), 70-88, 2017
Electrochemical and photochemical reduction of CO2, or a smart combination of both, are appealing approaches for the storage of renewable, intermittent energies and may lead to the production of fuels and of value-added chemicals. By using only earth-abundant metal (Cu, Ni, Co, Mn, Fe) complexes, cheap electrodes and/or cheap sacrificial electron donors and visible light sensitizers, systems functioning with molecular catalysts have been recently designed, showing promising results, in particular, for the two-electron reduction of the carbon dioxide. By combining experimental and mechanistic studies, key parameters controlling the catalysis efficiency have been deciphered, opening the way to the design of future, more efficient and durable catalysts, as well as to the development of electrochemical or photoelectrochemical cells, all being key steps for the emergence of applied devices. The most recent advances related to these issues are discussed in this review.
https://doi.org/10.1021/acscatal.6b02181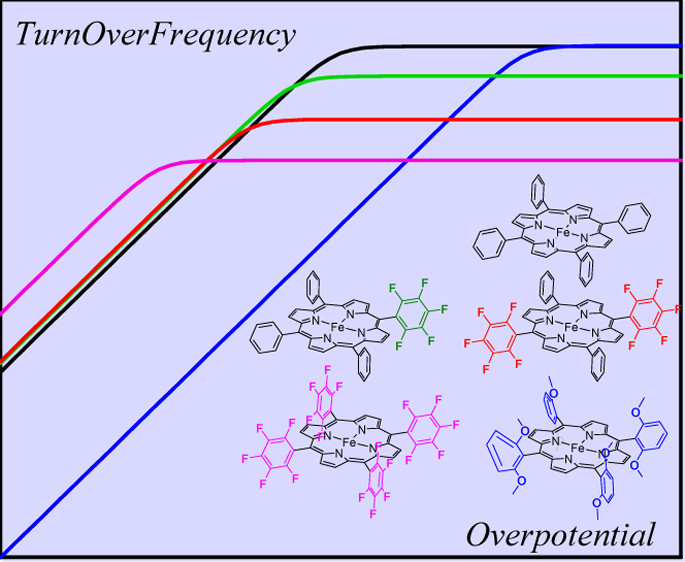
Dissection of Electronic Substituent Effects in Multielectron–Multistep Molecular Catalysis. Electrochemical CO2-to-CO Conversion Catalyzed by Iron Porphyrins
J. Phys. Chem. C 120 (51), 28951-28960, 2016
Redox pairs of transition metal complexes are often involved in small molecule activation in response to modern energy challenges as well as in other areas of electrocatalysis. Within such a family of molecular electrocatalysts, ligand substitution is a means of varying catalytic efficiency, best gauged through catalytic Tafel plots relating overpotential and turnover frequency. In practice, efficient molecular catalysis involves multielectron–multistep processes. It is in this framework that we discuss through-structure inductive substituent effects. What the best choice is for the reference thermodynamic index, how the global substituent effect may be expressed as a function of this index, and how it may be dissected into individual effects assigned to each of the reaction steps are challenging questions that are addressed and resolved here for the first time. The discussion is illustrated by the effect of successive phenyl perfluoration and of o,o′-methoxy substitution of the FeI/0 tetraphenylporphyrin catalysts of the CO2-to-CO electrochemical conversion. Consequences on the relative position of the catalytic Tafel plots are also examined. This analysis of through-structure electronic effects is a necessary preliminary to the investigation of substituent through-space effects (electrostatic, H-bonding) because, albeit of different nature, they may occur simultaneously. Investigation of these two aspects of substituent effects and of the rules that emerge thereof pave the way to future imaginative design of catalysts for the CO2-to-CO-conversion and also for any other molecular catalytic reactions.
https://doi.org/10.1021/acs.jpcc.6b09947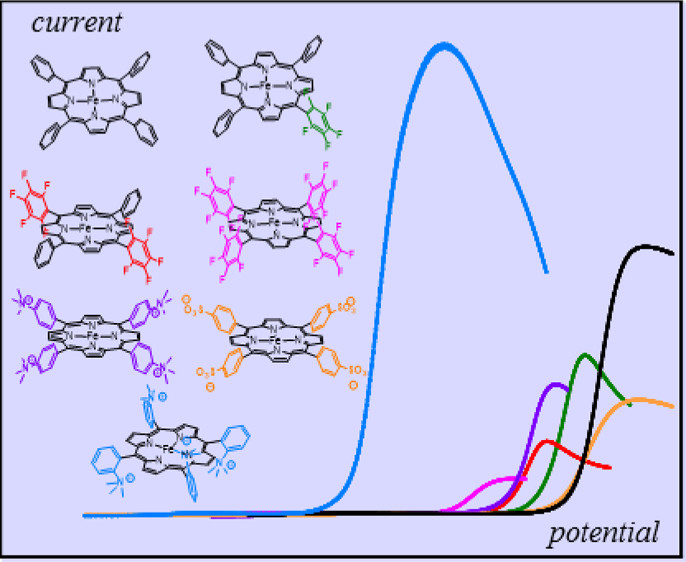
Through-Space Charge Interaction Substituent Effects in Molecular Catalysis Leading to the Design of the Most Efficient Catalyst of CO2-to-CO Electrochemical Conversion
J. Am. Chem. Soc. 138 (51), 16639-16644, 2016
The starting point of this study of through-space substituent effects on the catalysis of the electrochemical CO2-to-CO conversion by iron(0) tetraphenylporphyrins is the linear free energy correlation between through-structure electronic effects and the iron(I/0) standard potential that we established separately. The introduction of four positively charged trimethylanilinium groups at the para positions of the tetraphenylporphyrin (TPP) phenyls results in an important positive deviation from the correlation and a parallel improvement of the catalytic Tafel plot. The assignment of this catalysis boosting effect to the Coulombic interaction of these positive charges with the negative charge borne by the initial Fe0–CO2 adduct is confirmed by the negative deviation observed when the four positive charges are replaced by four negative charges borne by sulfonate groups also installed in the para positions of the TPP phenyls. The climax of this strategy of catalysis boosting by means of Coulombic stabilization of the initial Fe0–CO2 adduct is reached when four positively charged trimethylanilinium groups are introduced at the ortho positions of the TPP phenyls. The addition of a large concentration of a weak acid—phenol—helps by cleaving one of the C–O bonds of CO2. The efficiency of the resulting catalyst is unprecedented, as can be judged by the catalytic Tafel plot benchmarking with all presently available catalysts of the electrochemical CO2-to-CO conversion. The maximal turnover frequency (TOF) is as high as 106 s–1 and is reached at an overpotential of only 220 mV; the extrapolated TOF at zero overpotential is larger than 300 s–1. This catalyst leads to a highly selective formation of CO (practically 100%) in spite of the presence of a high concentration of phenol, which could have favored H2 evolution. It is also very stable, showing no significant alteration after more than 80 h of electrolysis.
https://doi.org/10.1021/jacs.6b07014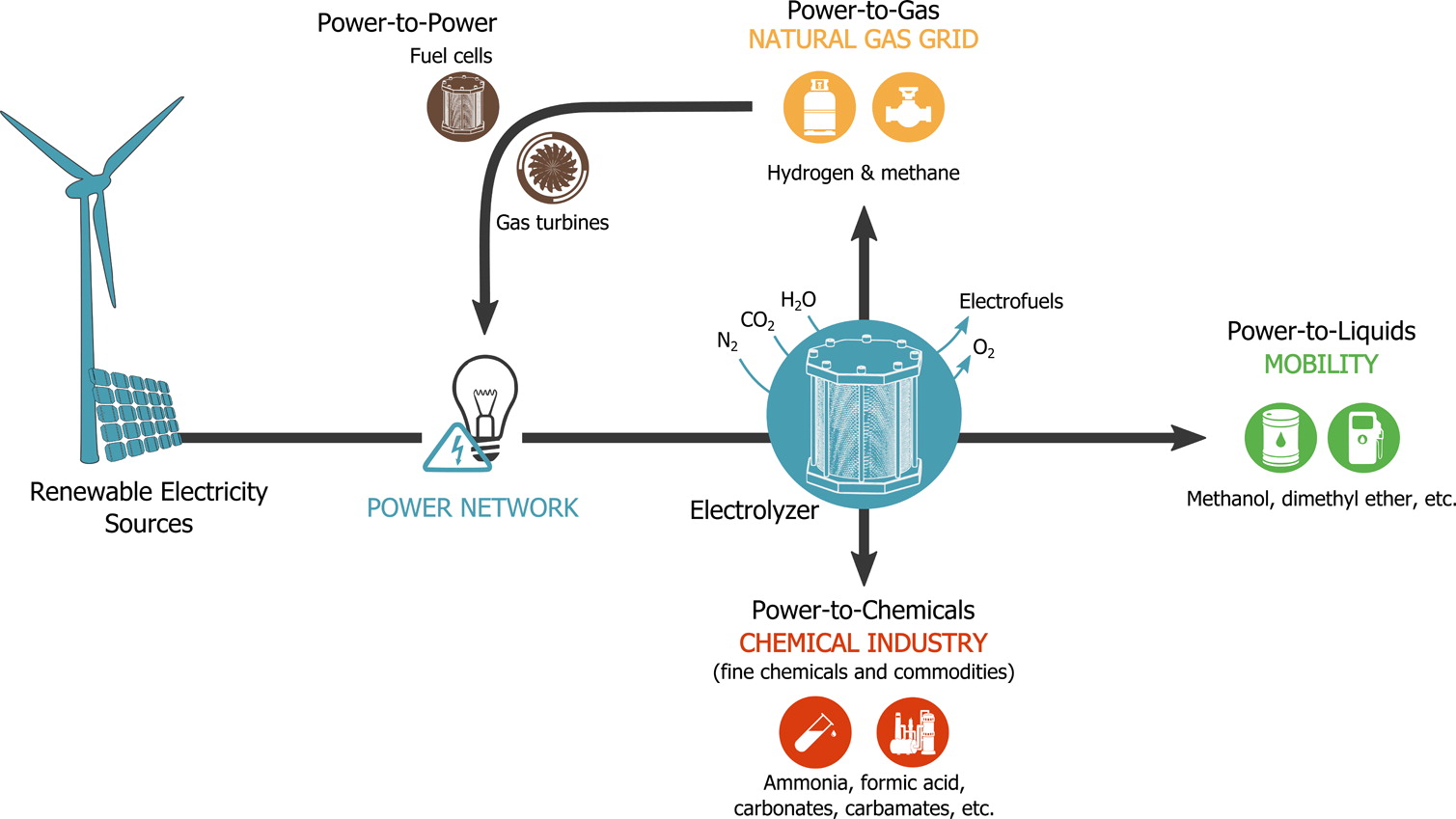
A Case for Electrofuels
ACS Energy Lett. 1 (5), 1062-1064, 2016
The United Nations conference on climate change (COP21) held last December in Paris illustrated public awareness regarding our current climate change and future energy issues. Solving these global challenges will involve various actions, from basic science to governmental regulations. In terms of technological development, sustainable scenarios aim at progressively replacing the fossil fuel economy that thrived during the 20th century. Yet, the lack of incentives and low price of oil are leaving the consumer with cheap and convenient technology solutions based on the combustion of fossil fuels. In this context, public research agencies should provide a long-term vision and endorse the funding for new, alternative routes.
https://doi.org/10.1021/acsenergylett.6b00510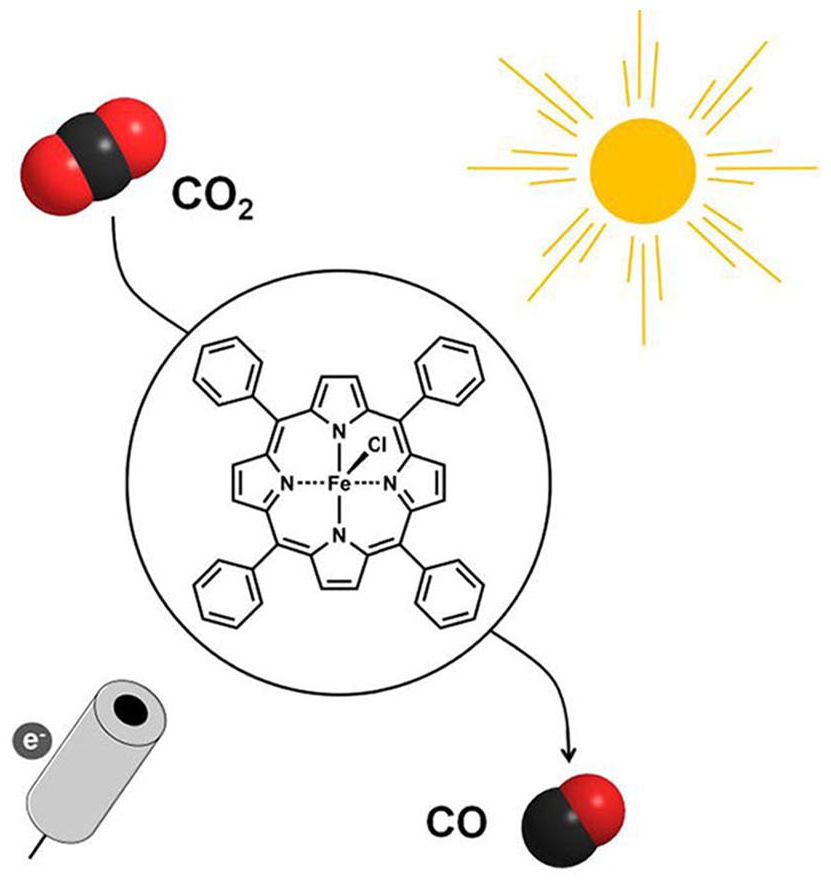
Molecular Catalysis of the Electrochemical and Photochemical Reduction of CO2 with Fe and Co Metal Based Complexes. Recent Advances
Coord. Chem. Rev. 334, 184-198, 2017
Reduction of CO2 is a fruitful approach for storage of renewable electric energy as well as for the production of value added chemicals. Using variously substituted iron tetraphenylporphyrins and iron and cobalt macrocyclic pentadendate N5 complexes electrochemically or photochemically reduced to their active states, selective and efficient molecular catalysis of the CO2-to-CO and of the CO2-to-HCOOH conversion was achieved at low overpotential. From fruitful joint experimental and mechanistic studies rooted in the analysis of cyclic voltammograms, the mechanisms for catalysis were fully deciphered, allowing not only to give a complete picture of the catalytic processes but also to identify the main parameters for optimizing the catalyst structure. Efficient CO2 catalysis was performed not only in aprotic but also in aqueous conditions, opening the way to the design of electrochemical or photoelectrochemical cells, a key step for future applied devices.
https://doi.org/10.1016/j.ccr.2016.09.005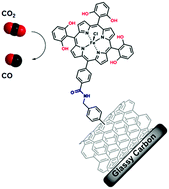
Catalytic CO2-to-CO Conversion in Water by Covalently Functionalized Carbon Nanotubes with a Molecular Iron Catalyst
Chem. Commun. 52 (81), 12084-12087, 2016
The covalent grafting of an Fe porphyrin on carbon nanotubes led to efficient electroreduction of CO2 into CO in water (pH 7.3). CO was obtained with high selectivity and turnover at 0.5 V overpotential. The grafting strategy may be further extended to various conductive and semi-conductive surfaces.
https://doi.org/10.1039/C6CC05430G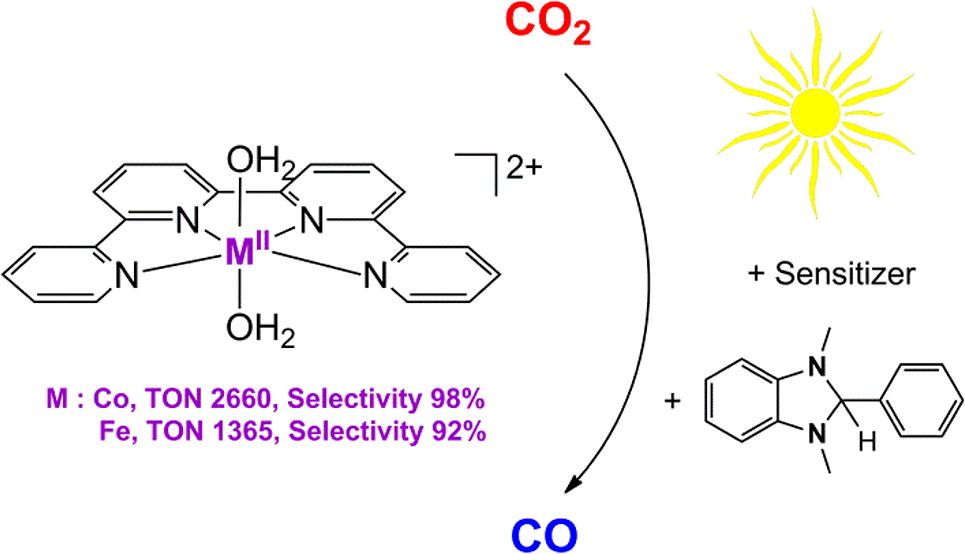
Highly Efficient and Selective Photocatalytic CO2 Reduction by Iron and Cobalt Quaterpyridine Complexes
J. Am. Chem. Soc. 138 (30), 9413-9416, 2016
The design of highly efficient and selective photocatalytic systems for CO2 reduction that are based on nonexpensive materials is a great challenge for chemists. The photocatalytic reduction of CO2 by [Co(qpy)(OH2)2]2+ (1) (qpy = 2,2′:6′,2″:6″,2‴-quaterpyridine) and [Fe(qpy)(OH2)2]2+ (2) have been investigated. With Ru(bpy)32+ as the photosensitizer and 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole as the sacrificial reductant in CH3CN/triethanolamine solution under visible-light excitation (blue light-emitting diode), a turnover number (TON) for CO as high as 2660 with 98% selectivity can be achieved for the cobalt catalyst. In the case of the iron catalyst, the TON was >3000 with up to 95% selectivity. More significantly, when Ru(bpy)32+ was replaced by the organic dye sensitizer purpurin, TONs of 790 and 1365 were achieved in N,N-dimethylformamide for the cobalt and iron catalysts, respectively.
https://doi.org/10.1021/jacs.6b06002
Running the Clock: CO2 Catalysis in the Age of Anthropocene
ACS Energy Lett. 1 (1), 281-282, 2016
The quest to escape the fossil fuel economy and its share of local and global pollution as well as geopolitical disruptions including large-scale migrations of millions of people has presented chemists with new challenges requiring the use of alternative, renewable energy sources. This quest has a name: solar fuels.
https://doi.org/10.1021/acsenergylett.6b00159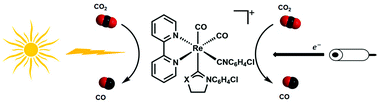
Photochemical and Electrochemical Catalytic Reduction of CO2 with NHC-containing Dicarbonyl Rhenium(I) Bipyridine Complexes
Dalton Trans. 45, 14524-14529, 2016
The electrochemical and photochemical catalytic reductions of CO2 using N,O and N,S-NHC-containing dicarbonyl rhenium(I) bipyridine complexes have been investigated. By replacing the carbonyl ligand in tricarbonyl rhenium(I) complexes with a weaker π-accepting ligand, the characteristic MLCT transitions shifted to lower energy. This makes photocatalysts capable of harvesting low-energy visible light for catalyzing CO2 reduction. A detailed study revealed that these dicarbonyl rhenium(I) complexes are also highly selective for photocatalysis of CO2 to CO with a good quantum efficiency (10%), similar to that of the tricarbonyl rhenium(I) complex analogues. From the electrochemical study, it was observed that the catalysts efficiently produce CO from CO2 with high turnover frequency and good stability over time.
https://doi.org/10.1039/c6dt01686c