Papers, communications and reviews… our recent published work is here.

Organic and Molecular Electrochemistry: Yes, Molecular Electrochemistry Can Do It
Curr. Opin. Electrochem. 15, A4-A5, 2019
The tools, methods and concepts of mechanistic molecular electrochemistry have been established for about four decades. They allowed the exploration of many aspects of organic, inorganic and bio reactivity in various areas ranging from (bio)chemical reactions and catalysis to (bio)analytical sciences. The current and vibrant renewal of electrochemistry, notably driven by contemporary energy challenges, has brought back on stage the use of fundamental molecular electrochemistry and stimulated new developments. The coupling of electrons, protons, and bond breaking or bond forming is a common feature of the catalytic processes that are emblematic of this renewal. These elementary steps, their degree of concertedness as well as their coupling with reactant and coreactant mass transport must be understood to drive the development of efficient processes. This issue illustrates the most prominent aspects of this remarkably active field.
https://www.sciencedirect.com/journal/current-opinion-in-electrochemistry/vol/15/suppl/C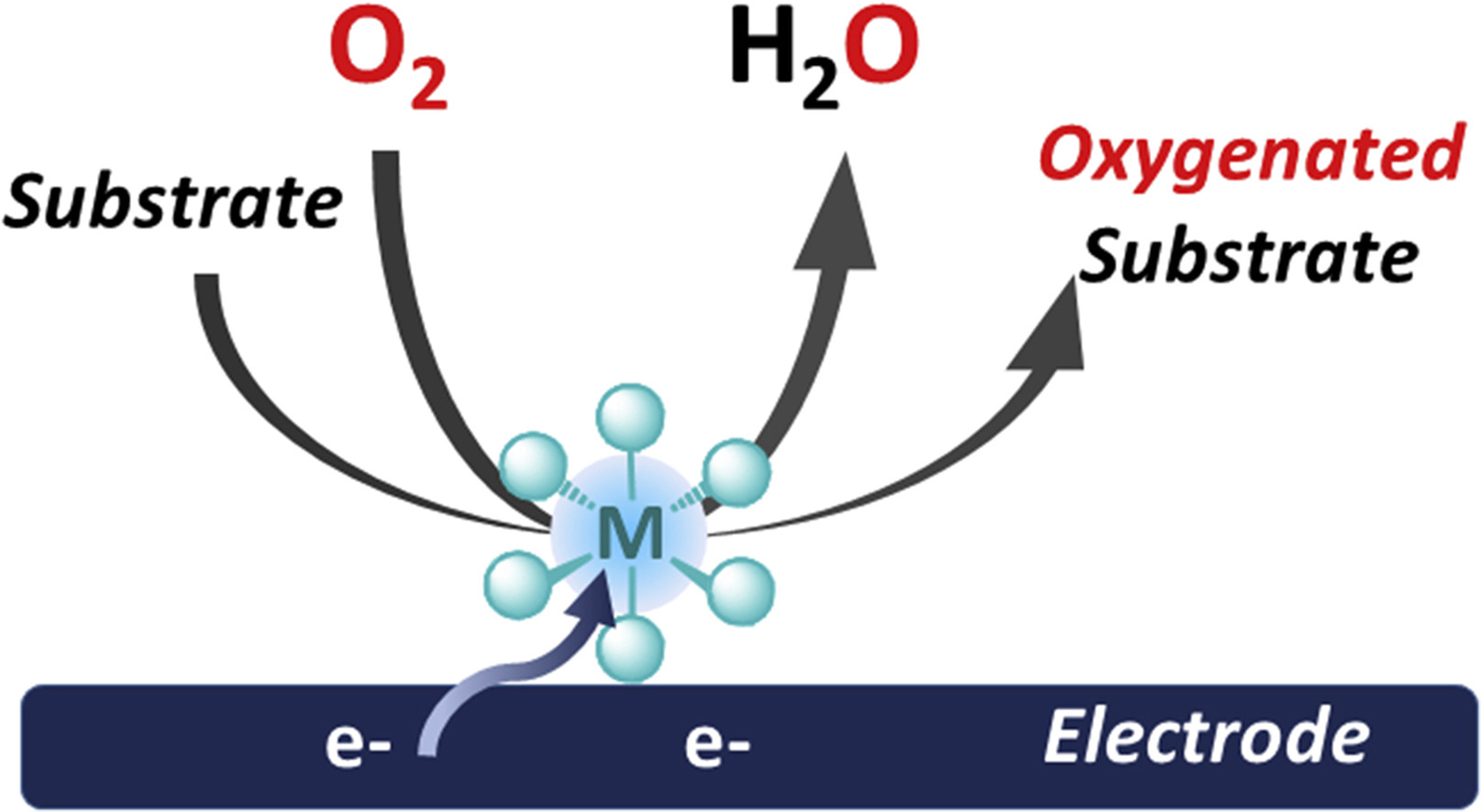
Bioinspired Molecular Catalysts for Homogenous Electrochemical Activation of Dioxygen
Curr. Opin. Electrochem. 15, 118-124, 2019
O2, which is abundant and environmentally benign, is the ideal green oxidant for oxidation reactions, which are key transformations in the chemical industry. Still, O2 needs to be activated, and this can be achieved through the so-called reductive activation of the O2 paradigm. Taking inspiration from metalloenzymes, where a non-noble redox active metal (iron, copper) controls the partial reduction of O2 via electron and proton transfers, metal-based synthetic systems can be developed to reproduce oxygenase activity. In the present article, we focus on fundamental aspects that serve as support for the development of 2-electron activation of O2 and generation of highly oxidizing metal-oxo species, thus paving the road for the development of electrocatalytic systems for organic substrate oxygenation. Scarce examples known in the literature capable of such reactivity and possible future developments are described.
https://doi.org/10.1016/j.coelec.2019.05.002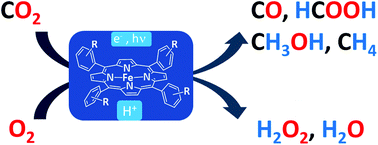
Small-Molecule Activation with Iron Porphyrins using Electrons, Photons and Protons: Some Recent Advances and Future Strategies
Dalton Trans. 48, 5869-5878, 2019
Substituted tetraphenyl Fe porphyrins are versatile molecular catalysts for the activation of small molecules (such as O2, H+ or CO2), which could lead to renewable energy storage, the direct production of fuels or new catalytic relevant processes. Herein, we review the recent studies of these earth-abundant metal catalysts for the electrochemical activation of dioxygen on the one hand and for the photostimulated reduction of carbon dioxide on the other hand. These two prototype reactions illustrate how mechanistic studies are the only rational approach to gain fundamental insights into the elementary steps that drive the catalysis and for identification of the key intrinsic parameters controlling the reactivity, offering in turn the possibility to rationally tune the structure of the catalysts as well as the catalytic conditions.
http://dx.doi.org/10.1039/c9dt00136k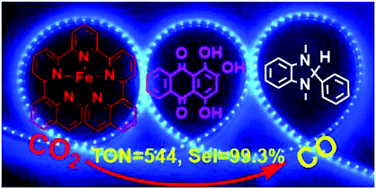
A molecular noble metal-free system for efficient visible light-driven reduction of CO2 to CO
Dalton Trans. 48, 9596-9602, 2019
A new pentadentate quinoline–pyridine ligand and its iron (1), cobalt (2) and nickel (3) complexes have been synthesized and characterized. The iron complex exhibits excellent photocatalytic activity towards CO2-to-CO conversion with a TON(CO) of 544 and a selectivity of 99.3% using the commercially available organic dye purpurin as the photosensitiser and BIH as the electron donor. In contrast, the cobalt and nickel complexes result in very low activity for CO production with a TON of only 8 and 15, respectively. On the other hand, all the three complexes show good electrocatalytic activity for CO2 reduction when using 2,2,2-trifluoroethanol as the proton source with the active intermediate of M0 species. The lack of activity in photocatalytic CO2 reduction by 2 and 3 can be attributed to the redox potential of MI/M0 which is significantly more negative than that of PP−/PP2− while in the case of 1 the FeI/Fe0 redox potential becomes more positive than that of the PP−/PP2− couple.
https://doi.org/10.1039/c9dt00425d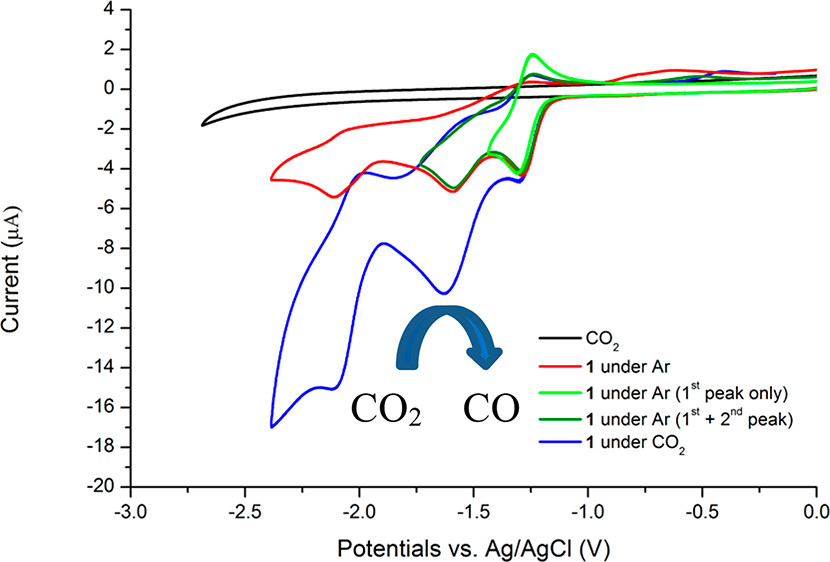
Electrochemical and Photochemical Reduction of CO2 Catalyzed by Re(I) Complexes Carrying Local Proton Sources
Organometallics 38, 1351−1360, 2019
The novel rhenium complexes fac-Re(pdbpy)(CO)3Cl (pdbpy = 4-phenyl-6-(phenyl-2,6-diol)-2,2′-bipyridine), 1, and fac-Re(ptbpy)(CO)3Cl (ptbpy = 4-phenyl-6-(phenyl-3,4,5-triol)-2,2′-bipyridine), 2, have been synthesized, and the single crystal X-ray diffraction (SC-XRD) structure of 1 was solved. The electrochemical behaviors of the complexes in acetonitrile under Ar and their catalytic performances for CO2 reduction with added water and MeOH are discussed. A detailed IR spectroelectrochemical study under Ar and CO2 atmospheres coupled with DFT calculations allows the identification of reduced species and the interpretation of the reduction mechanisms. Comparison between the rhenium complexes and the corresponding Mn derivatives Mn(pdbpy)(CO)3Br, 3, and Mn(ptbpy)(CO)3Br, 4, has been also considered. Finally, photostimulated conversion of the CO2 was investigated with catalysts (1, 3, and 4) under visible light irradiation (λ > 420 nm) in acetonitrile as solvent. Remarkably, 1 and 3 catalysts were active toward CO2, producing formate with good selectivity and turnover number (TON). For example, 3 gives 62% selectivity for HCOO– and a TON of 80, and Re compound 1 gives 74% selectivity for HCOO– and a TON of 86.
https://doi.org/10.1021/acs.organomet.8b00588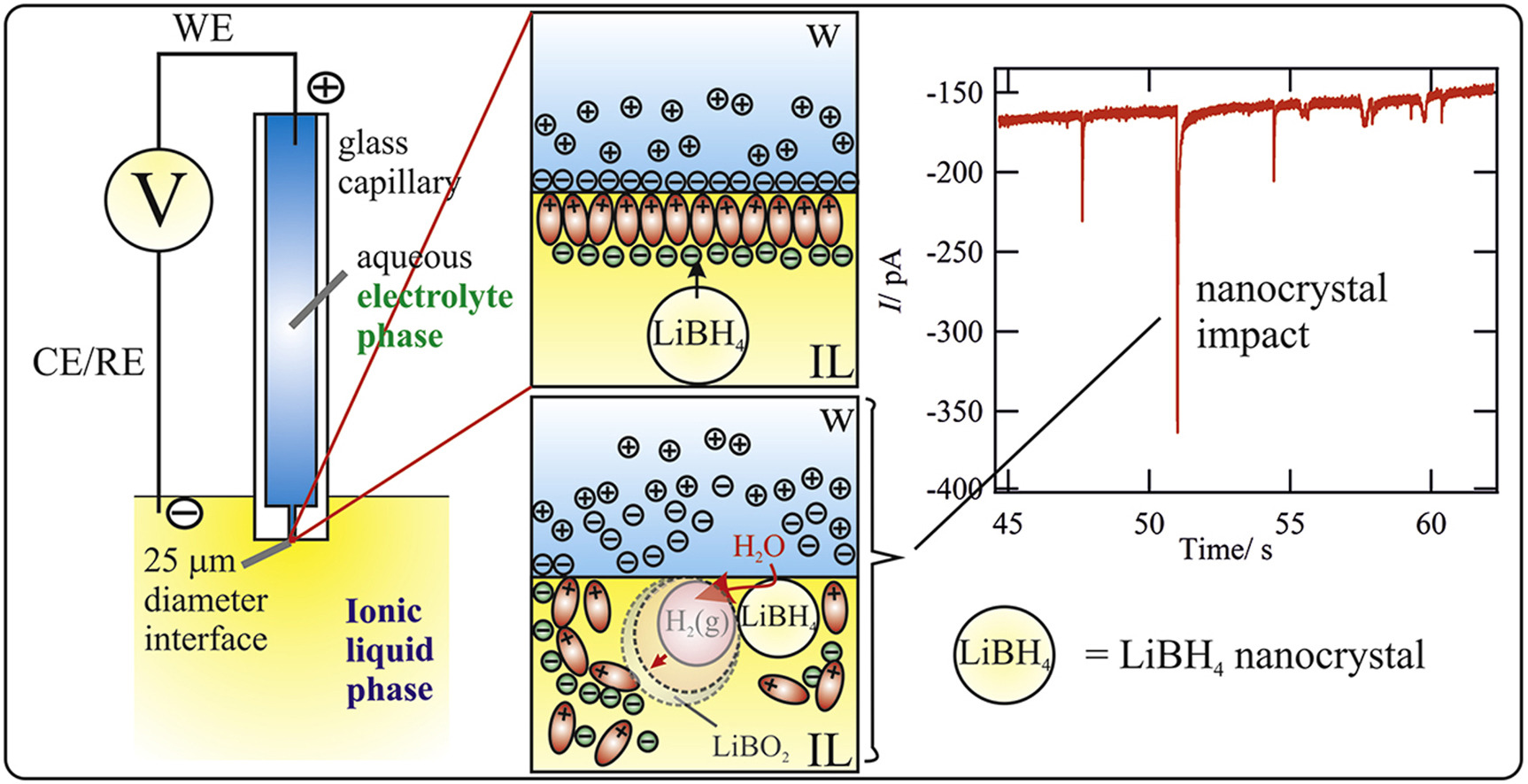
Single LiBH4 Nanocrystal Stochastic Impacts at a Micro Water | Ionic Liquid Interface
Electrochimica Acta 229, 220-230, 2019
LiBH4 is often employed as a reducing agent for metal nanoparticle (NP) preparation but is inherently a solid-state H2 hydrogen storage agent. Herein it is shown, through a combination of electron/optical microscopies and single entity electrochemical study, that LiBH4 is stored in the solid state within an ionic liquid (IL) as nanocrystals (NCs). The electrochemical monitoring of an immiscible water|IL (w|IL) micro-liquid|liquid interface (LLI) shows interfacial charge exchange associated with the stochastic impacts of single NCs. Meanwhile, in situ optical monitoring of a w|metal or w|IL interface shows that such impacts are associated with the development of a H2-in-IL micro/nano-foam related to the poor solubility of H2. Both the presence of solid NCs and the latter H2-in-IL foam suggest that H2 release from LiBH4-in-IL is a slow, but likely controlled process. The rate of H2 production at a macroscopic LLI is further confirmed by gas chromatographic measurements, in very good agreement with microscopic observations. The electrochemical LLI provides unique investigative access to LiBH4 NCs and offers insight into H2 storage in ILs, or for direct borohydride fuel cells, as well as NP synthesis.
https://www.sciencedirect.com/science/article/pii/S0013468618328123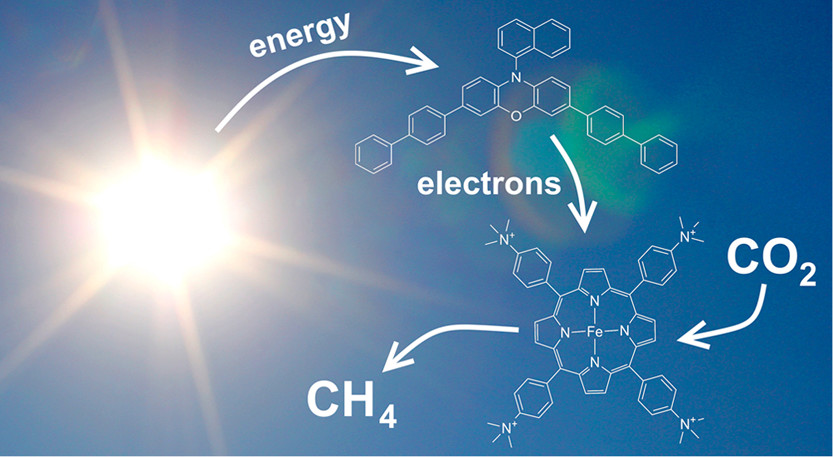
Visible-Light-Driven Conversion of CO2 to CH4 with an Organic Sensitizer and an Iron Porphyrin Catalyst
J. Am. Chem. Soc. 140, 17830−17834, 2018
Using a phenoxazine-based organic photosensitizer and an iron porphyrin molecular catalyst, we demonstrated photochemical reduction of CO2 to CO and CH4 with turnover numbers (TONs) of 149 and 29, respectively, under visible-light irradiation (λ > 435 nm) with a tertiary amine as sacrificial electron donor. This work is the first example of a molecular system using an earth-abundant metal catalyst and an organic dye to effect complete 8e–/8H+ reduction of CO2 to CH4, as opposed to typical 2e-/2H+ products of CO or formic acid. The catalytic system continuously produced methane even after prolonged irradiation up to 4 days. Using CO as the feedstock, the same reactive system was able to produce CH4 with 85% selectivity, 80 TON and a quantum yield of 0.47%. The redox properties of the organic photosensitizer and acidity of the proton source were shown to play a key role in driving the 8e-/8H+ processes.
https://doi.org/10.1021/jacs.8b09740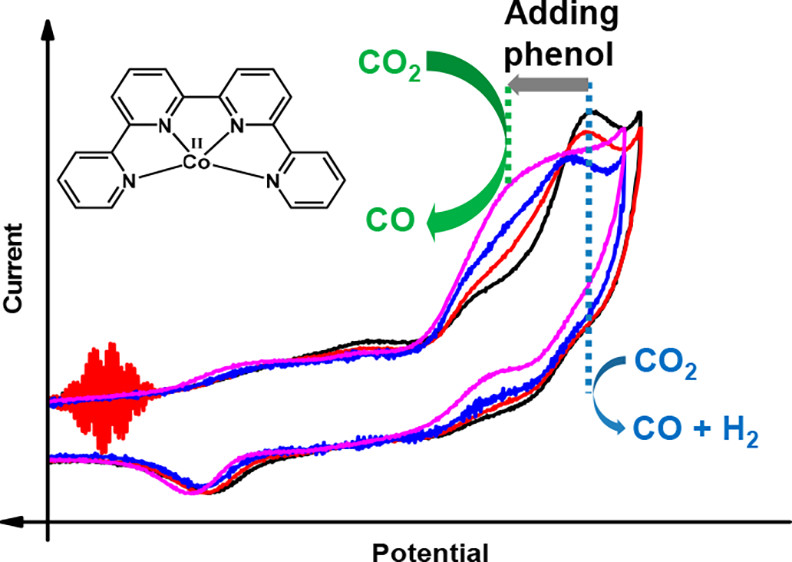
Molecular Electrochemical Catalysis of the CO2-to-CO Conversion with a Co Complex: A Cyclic Voltammetry Mechanistic Investigation
Organometallics 38, 1280−1285, 2019
The electrochemical catalytic reduction of CO2 into CO could be achieved with excellent selectivity and rate in acetonitrile in the presence of phenol with cobalt 2,2′:6′,2″:6″,2‴-quaterpyridine complex [CoII(qpy)(H2O)2]2+ (Co) acting as a molecular catalyst. Upon using cyclic voltammetry at low and high scan rate (up to 500 V/s) two catalytic pathways have been identified. At a low concentration of phenol (<1 M), catalysis mainly occurs after the reduction of Co with three electrons. In that case, the selectivity for CO production is ca. 80% with 20% of H2 as by product, along with a turnover frequency of 1.2 × 104 s–1 for CO production at an overpotential η of ca. 0.6 V. The triply reduced active species binds to CO2 and the C–O bond is cleaved thanks to the acid. At very large concentration of phenol (3 M), another pathway becomes predominant: the doubly reduced species binds to CO2, while its reductive protonation leads to CO formation. As already shown, this later process is endowed with fast rate at low overpotential (turnover frequency of 3 × 104 s–1 at η = 0.3 V) and 95% selectivity for CO production. By varying the phenol concentration and the scan rate in voltammetry experiments, it was thus possible to identify, activate, and characterize several pathways for the CO2-to-CO conversion and to decipher Co electrochemical reactivity toward CO2.
https://doi.org/10.1021/acs.organomet.8b00555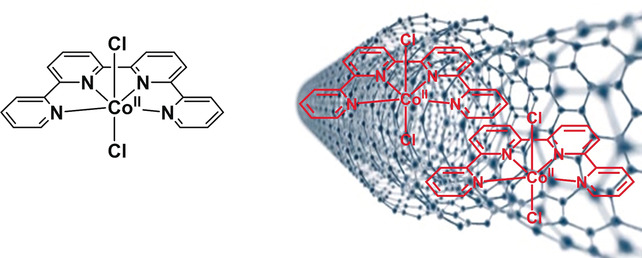
A Hybrid Co Quaterpyridine Complex/Carbon Nanotube Catalytic Material for CO2 Reduction in Water
Angew. Chem. Int. Ed. 57, 7769-7773, 2018
Associating a metal‐based catalyst to a carbon‐based nanomaterial is a promising approach for the production of solar fuels from CO2. Upon appending a CoII quaterpyridine complex [Co(qpy)]2 at the surface of multi‐walled carbon nanotubes, CO2 conversion into CO was realized in water at pH 7.3 with 100 % catalytic selectivity and 100 % Faradaic efficiency, at low catalyst loading and reduced overpotential. A current density of 0.94 mA cm−2 was reached at −0.35 V vs. RHE (240 mV overpotential), and 9.3 mA cm−2 could be sustained for hours at only 340 mV overpotential with excellent catalyst stability (89 095 catalytic cycles in 4.5 h), while 19.9 mA cm−2 was met at 440 mV overpotential. Such a hybrid material combines the high selectivity of a homogeneous molecular catalyst to the robustness of a heterogeneous material. Catalytic performances compare well with those of noble‐metal‐based nano‐electrocatalysts and atomically dispersed metal atoms in carbon matrices.
https://dx.doi.org/10.1002/anie.201802792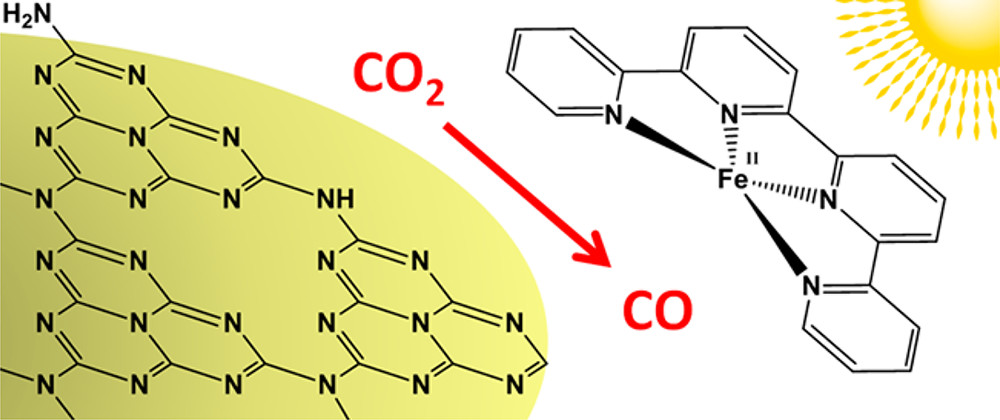
A Carbon Nitride/Fe Quaterpyridine Catalytic System for Photostimulated CO2‑to-CO Conversion with Visible Light
J. Am. Chem. Soc. 140, 7437−7440, 2018
Efficient and selective photostimulated CO2-to-CO reduction by a photocatalytic system consisting of an iron-complex catalyst and a mesoporous graphitic carbon nitride (mpg-C3N4) redox photosensitizer is reported for the first time. Irradiation in the visible region (λ ≥ 400 nm) of an CH3CN/triethanolamine (4:1, v/v) solution containing [Fe(qpy)(H2O)2]2+ (qpy = 2,2′:6′,2′′:6′′,2′′-quaterpyridine) and mpg-C3N4 resulted in CO evolution with 97% selectivity, a turnover number of 155, and an apparent quantum yield of ca. 4.2%. This hybrid catalytic system, comprising only earth abundant elements, opens new perspectives for solar fuels production using CO2 as a renewable feedstock.
https://doi.org/10.1021/jacs.8b04007