Papers, communications and reviews… our recent published work is here.
Best practices for experiments and reporting in photocatalytic CO2 reduction
Nature Catal. 6, 657-665, 2023
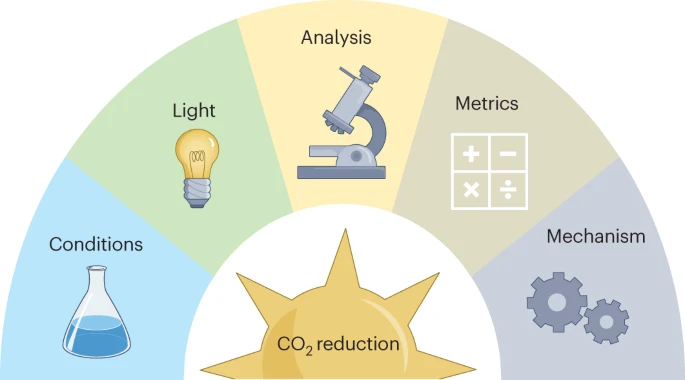
Visible-light-driven conversion of CO2 to fuels and valuable compounds has experienced tremendous activity in recent years, aiming at storing solar energy into chemical bonds using CO2 as a renewable feedstock, ultimately at massive scale. Despite these efforts, processes and catalytic systems are still at an early stage of development, with fundamental mechanistic challenges as pre-requisites for device design. In this context, collective efforts currently necessitate the exploration of a variety of approaches. On the other hand, an alignment of practices is required to ensure robustness, precision and accuracy of the results, as well as shared metrics and tools for advancing our understanding of the necessary processes. This Perspective aims to provide guidelines and a framework towards these objectives.
https://doi.org/10.1038/s41929-023-00992-7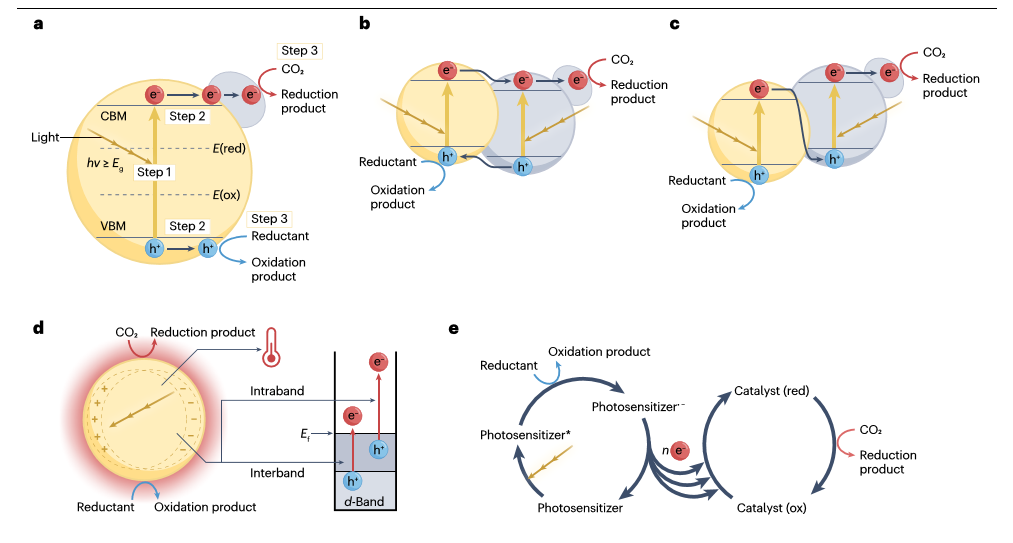
Photocatalytic CO2 reduction
Nat. Rev. Methods Primers 3, 61, 2023
Using sunlight to power CO2 conversion into value-added chemicals and fuels is a promising technology to use anthropogenic CO2 emissions for alleviating our dependence on fossil fuels. In this Primer, we provide a holistic step-by-step guide for the experimentation of photocatalytic CO2 reduction, including catalyst synthesis and characterization, reactor construction, photocatalytic testing and mechanism exploration. We compare and analyse the state-of-the-art results with different photocatalysts and discuss possible reaction mechanisms. Furthermore, important considerations regarding practical application of photocatalytic CO2 reduction are highlighted and strategies to enhance energy conversion efficiency and product selectivity are summarized. This Primer also reveals current issues of reproducibility, standardizes data reporting and proposes a unified operation condition. Finally, future directions are outlined in terms of experiments, calculations, big-data development and practical application.
https://doi.org/10.1038/s43586-023-00243-w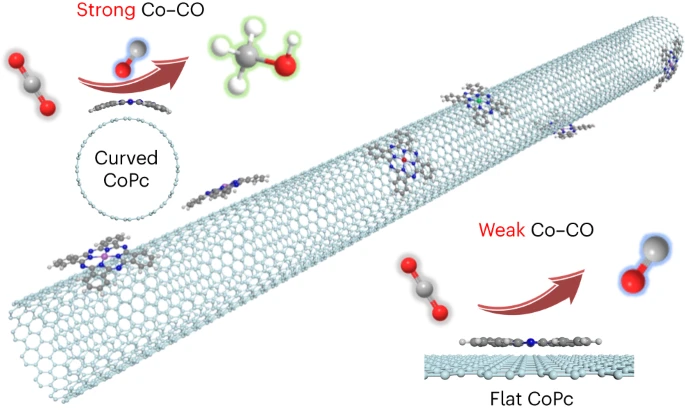
Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports
Nat. Catal. 6, 818-828, 2023
Support-induced strain engineering is useful for modulating the properties of two-dimensional materials. However, controlling strain of planar molecules is technically challenging due to their sub-2 nm lateral size. Additionally, the effect of strain on molecular properties remains poorly understood. Here we show that carbon nanotubes (CNTs) are ideal substrates for inducing optimum properties through molecular curvature. In a tandem-flow electrolyser with monodispersed cobalt phthalocyanine (CoPc) on single-walled CNTs (CoPc/SWCNTs) for CO2 reduction, we achieve a methanol partial current density of >90 mA cm−2 with >60% selectivity, surpassing wide multiwalled CNTs at 16.6%. We report vibronic and X-ray spectroscopies to unravel the distinct local geometries and electronic structures induced by the strong molecule–support interactions. Grand canonical density functional theory confirms that curved CoPc/SWCNTs improve *CO binding to enable subsequent reduction, whereas wide multiwalled CNTs favour CO desorption. Our results show the important role of SWCNTs beyond catalyst dispersion and electron conduction.
https://doi.org/10.1038/s41929-023-01005-3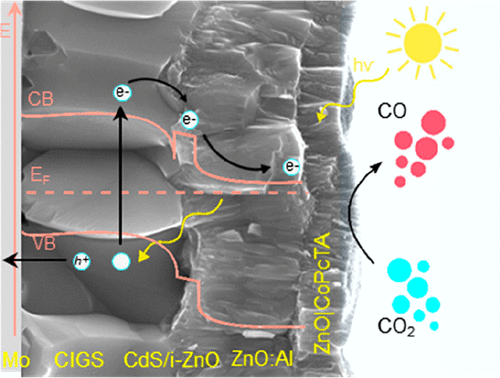
Multifunctional Photovoltaic Window Layers for Solar-Driven Catalytic Conversion of CO2: The Case of CIGS Solar Cells
ACS Energy Lett. 8, 3488-3493, 2023
Using a fast and simple one-step electrochemical method, we developed transparent and conductive ZnO nanoporous layers encapsulating molecular catalysts, showcasing dual functionality as a window layer for thin-film solar cells and a catalytic layer for solar-to-fuel conversion. As a proof of concept, tetraammonium-substituted Co phthalocyanine (CoPcTA) was encapsulated into the window layer of high-efficiency Cu(In,Ga)Se2 (CIGS) solar cells demonstrating photoelectrochemical (PEC) reduction of CO2 into CO with a selectivity of 93% and current densities up to ca. 7 mA cm–2 at −1.7 V vs SCE under 1 sun irradiation, which corresponds to a turnover number (TON) of above 100000 and a turnover frequency (TOF) of 10 s–1 after 3 h. The simplicity and versatility of this approach make the nanoporous catalytic ZnO layer not only easily adaptable to different high-efficiency solar cells but also pave the way for flexible testing of diverse molecular catalysts for CO2 conversion into diverse, valuable fuels.
https://doi.org/10.1021/acsenergylett.3c01205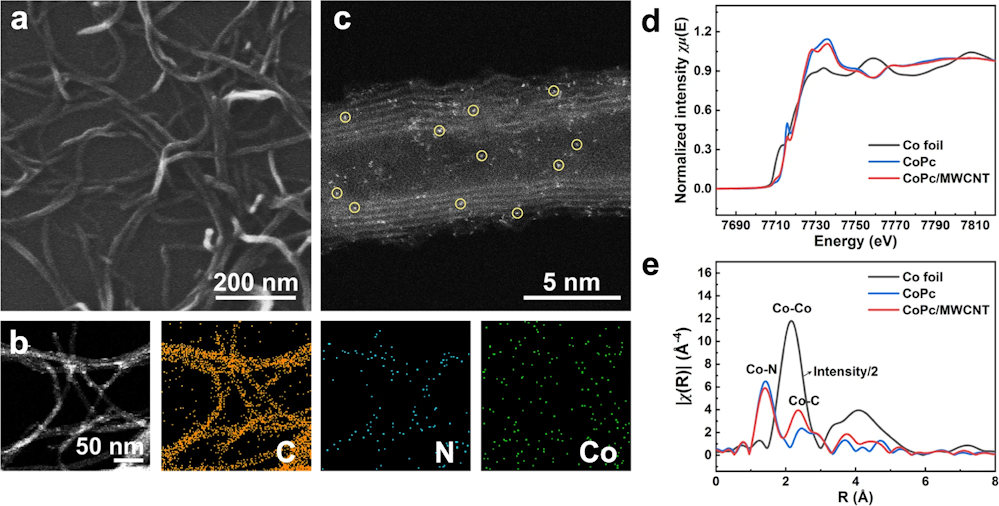
In-situ spectroscopic probe of the intrinsic structure feature of single-atom center in electrochemical CO/CO2 reduction to methanol
Nature Commun. 14, 3401, 2023
While exploring the process of CO/CO2 electroreduction (COxRR) is of great significance to achieve carbon recycling, deciphering reaction mechanisms so as to further design catalytic systems able to overcome sluggish kinetics remains challenging. In this work, a model single-Co-atom catalyst with well-defined coordination structure is developed and employed as a platform to unravel the underlying reaction mechanism of COxRR. The as-prepared single-Co-atom catalyst exhibits a maximum methanol Faradaic efficiency as high as 65% at 30 mA/cm2 in a membrane electrode assembly electrolyzer, while on the contrary, the reduction pathway of CO2 to methanol is strongly decreased in CO2RR. In-situ X-ray absorption and Fourier-transform infrared spectroscopies point to a different adsorption configuration of *CO intermediate in CORR as compared to that in CO2RR, with a weaker stretching vibration of the C–O bond in the former case. Theoretical calculations further evidence the low energy barrier for the formation of a H-CoPc-CO– species, which is a critical factor in promoting the electrochemical reduction of CO to methanol.
https://doi.org/10.1038/s41467-023-39153-6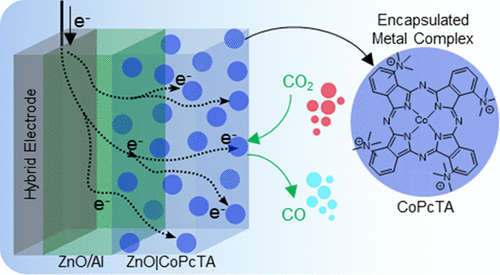
Transparent porous ZnO|metal complexes nanostructured materials: application to electrocatalytic CO2 reduction
ACS Appl. Nano Mater. 6, 10625-10636, 2023
We developed a simple and versatile approach for the electrochemical growth of hybrid ZnO|molecular catalyst nanostructured layers. Metal oxide|catalyst hybrid nanoporous layers with a sponge-like structure at the nanoscale and multiscale three-dimensional (3D) hierarchical structures based on nanoporous zinc oxide (ZnO) layers grown on ZnO nanorods were obtained. The thickness and structure of the hybrid nanoporous layers as well as the catalyst concentration can be tuned. This method allows the introduction of water-soluble molecular catalysts based on porphyrin and/or phthalocyanine derivatives into the ZnO matrix with a homogeneous distribution of the complex into the material. As an illustrative example, combining hybrid ZnO with a very low concentration of an encapsulated Co-based molecular catalyst inside the oxide layer results in a 97% catalytic response toward CO2 reduction to CO with large currents in an organic solvent, highlighting the excellent electrocatalytic activities of such layers, which combine porosity, electronic conductivity, and synergetic properties from its components.
https://doi.org/10.1021/acsanm.3c01591
Predicting ruthenium catalysed hydrogenation of esters using machine learning
Digital Discovery 2, 819-827, 2023
Catalytic hydrogenation of esters is a sustainable approach for the production of fine chemicals, and pharmaceutical drugs. However, the efficiency and cost of catalysts are often bottlenecks in the commercialization of such technologies. The conventional approach to catalyst discovery is based on empiricism, which makes the discovery process time-consuming and expensive. There is an urgent need to develop effective approaches to discover efficient catalysts for hydrogenation reactions. In this work, we explore the approach of machine learning to predict outcomes of catalytic hydrogenation of esters using various ML architectures – NN, GP, decision tree, random forest, KNN, and linear regression. Our optimized models can predict the reaction yields with reasonable error for example, a root mean square error (RMSE) of 11.76% using GP on unseen data and suggest that the use of certain chemical descriptors (e.g. electronic parameters) selectively can result in a more accurate model. Furthermore, studies have also been carried out for the prediction of catalysts and reaction conditions such as temperature and pressure as well as their validation by performing hydrogenation reactions to improve the poor yields described in the dataset.
https://doi.org/10.1039/D3DD00029J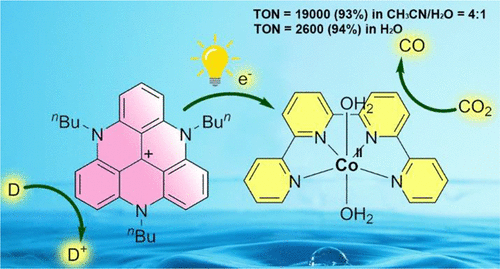
Light-driven reduction of CO2 to CO in water with a molecular cobalt catalyst and an organic sensitizer
ACS Catal. 13, 5979-5985, 2023
We report an efficient visible light-driven CO2 reduction system that functions in water and without any noble metal nor rare materials. Using the cobalt complex [Co(qpy)(OH2)2]2+ (1, qpy = 2,2′:6′,2″:6″,2‴-quaterpyridine) as a catalyst, an organic triazatriangulenium (TATA+) salt as the photosensitizer (PS), BIH + TEOA (BIH = 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole and TEOA = triethanolamine) as the sacrificial reductant (SD), CO and formate were first produced with a total TON >3700 upon irradiation in CO2-saturated CH3CN solution with visible light. Upon the addition of a weak Brönsted acid (water), catalysis was enhanced and directed toward CO production (19,000 TON, 93% selectivity). The photocatalytic system was further shown to function in pure water as a solvent. High metrics with a TON for CO of 2600 and 94% selectivity were obtained using TEA (triethylamine) as the SD.
https://doi.org/10.1021/acscatal.3c00036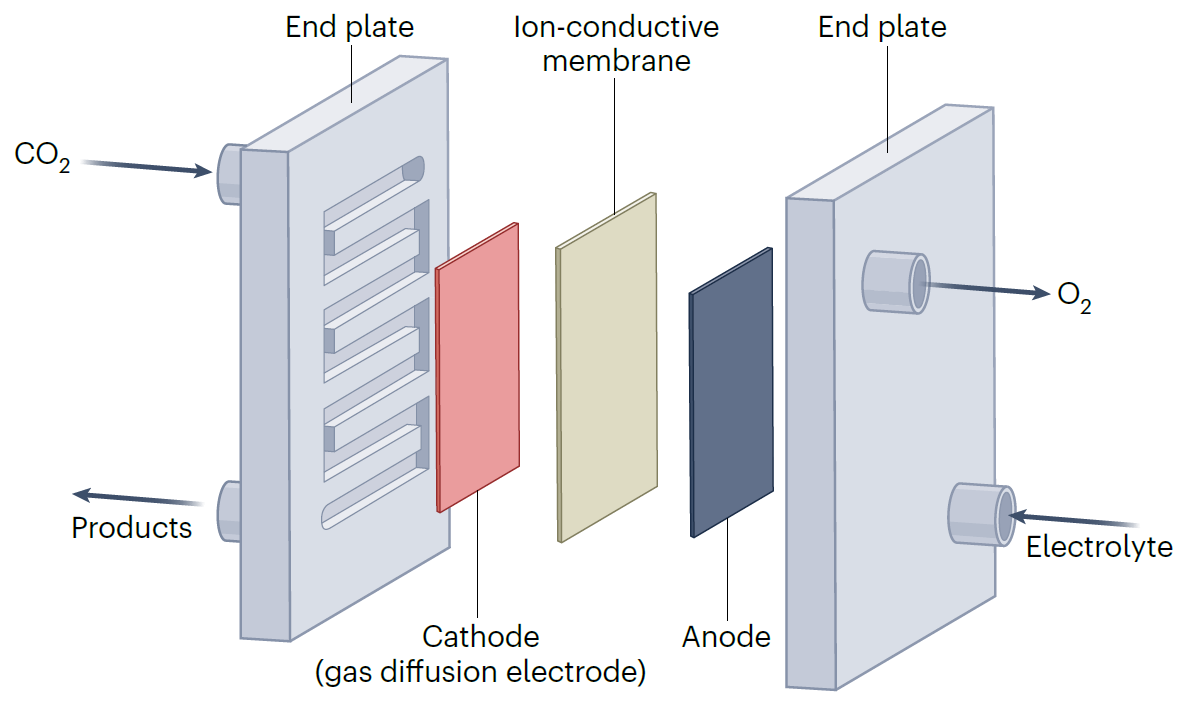
Best practices for electrochemical reduction of carbon dioxide
Nature Sustain. 6, 236–238, 2023
Carbon capture, utilization and storage, a fundamental process to a sustainable future, relies on a suite of technologies among which electrochemical reduction of carbon dioxide is essential. Here, we discuss the issues faced when reporting performance of this technology and recommend how to move forward at both materials and device levels.
https://doi.org/10.1038/s41893-022-01034-z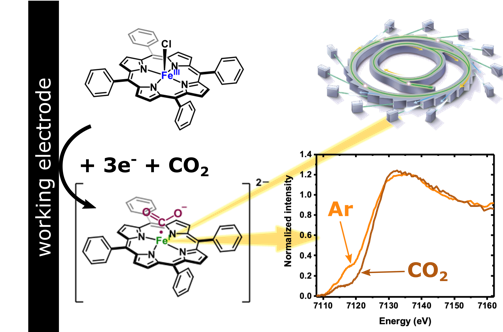
In situ X-ray absorption spectroscopy in homogeneous conditions reveals interactions between CO2 and a doubly and triply reduced iron(III) porphyrin, then leading to catalysis
ChemCatChem, e202201298, 2023
Iron porphyrins are attractive catalysts for the electrochemical reduction of carbon dioxide (CO2), owing to their high activity and selectivity, while being tunable through ligand functionalization. Iron tetraphenyl porphyrin (FeTPP) is the simplest of them and its catalytic behavior towards CO2 has been studied for decades. Although kinetic information is available, spectroscopic signatures are lacking to describe intermediate species along the catalytic cycle. We have used in situ UV-Visible and X-ray absorption near edge spectroscopy (XANES) to monitor the local and electronic structure of FeTPP homogeneously disolved in dimethyl formamide (DMF) under reductive potentials. We identify the Fe(III) starting species, together with its one, two and three electron reduced counterparts under both argon and CO2 atmospheres. Under argon, the second and third reductions lead to species with electronic density shared between the metal and the porphyrin backbone. In the presence of CO2 and with a low amount of protons, the doubly and triply reduced species interact with CO2 at the metallic site. In light of these results, we propose an electronic structure for a key intermediate along the catalytic cycle of the CO2-to-CO reduction reaction.
https://doi.org/10.1002/cctc.202201298