Running the clock, catalytic reduction of small molecules such as CO2, N2 and O2
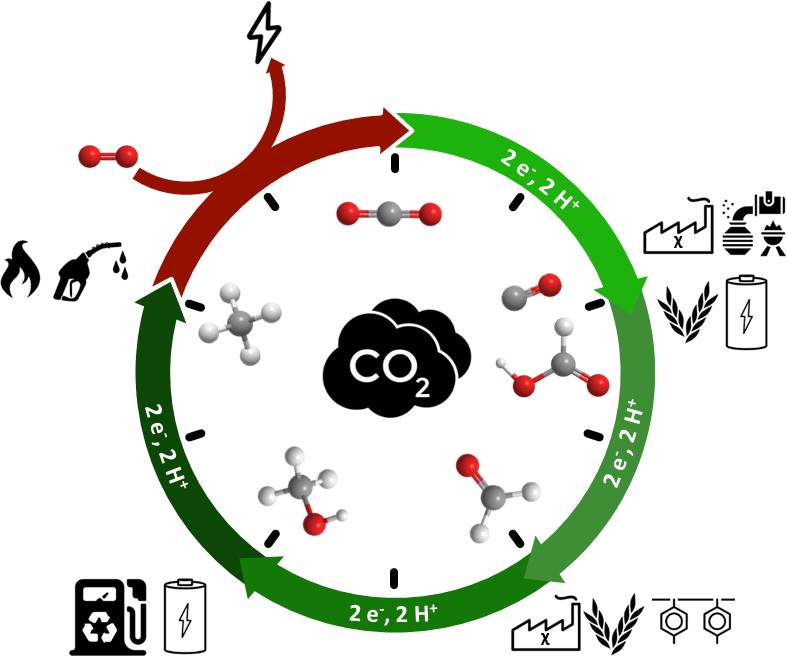
Activation of small molecules is a field of intense activity due to promising applications in energy storage, renewable fuels production, green chemistry and climate change mitigation. For example, carbon dioxide, one of the most important greenhouse gases in the atmosphere, is chemically inert, but it is possible to use it as a renewable feedstock and to transform it into fuels or useful products such as polymers. Understanding and controlling the transformation of such molecules are keys toward inventing scalable technologies that will lead to massive use of renewable energies to power the planet as well as to give energy access to everyone.
In an effort to contribute to these goals, we develop an original strategy combining spectroscopic, electrochemical and photochemical tools, methods and concepts to study the mechanistic aspects of reduction of CO2, N2 and O2, using earth abundant metal complexes either.
It led us to explore and model proton-coupled electron transfer reactivity in chemistry, to contribute to the theoretical analysis of electrochemical catalytic cyclic voltammetry responses allowing to benchmark molecular catalysts on rational ground. These fundamental studies in turn fuel the discovery and synthesis of new active catalysts, leading to unprecedented reactions such as CO2 electrochemical reduction to methanol with a Co complex or CO2 visible light driven conversion to methane with an Fe porphyrin.
CO2 catalytic reduction
Based on mechanistic studies mainly rooted in cyclic voltammetry, we have been developing earth abundant metal based catalysts able to reduce CO2 selectively and with high efficiency, such as Fe porphyrins, Co phthalocyanines and quaterpyridines. These catalysts may be used for the electrochemical conversion of CO2, either homogeneously dispersed in solution or deposited into thin films at the surface of conductive or semi-conductive electrodes. Alternatively, visible light driven conversion of the CO2 using appropriate sensitizer and electron donor also leads to remarkable catalytic activity.
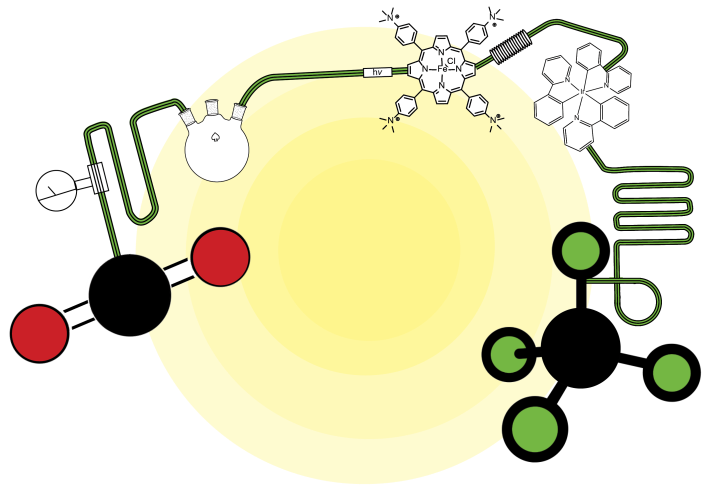
We are currently developing multimetallic molecular catalysts and exploiting cooperative effects between the metals, as performed by some natural enzymes.
In parallel we develop lab-scale electrolyzers and photo-electrolyzers capable of converting carbon dioxide. This work is done in collaboration with industrial partners and paves the way to scalable renewable energy storage technologies using CO2 as a renewable feedstock.
N2 catalytic reduction
Conversion of nitrogen into ammonia is one of the most important industrial process, leading notably to nitrogen fertilizers. To understand these multi proton-coupled electron transfer processes, we are combining electrochemical and spectroscopic techniques. It drives us to the design of Fe and Mo molecular complexes able to efficiently catalyse the conversion of N2 in mild conditions at conductive electrodes.
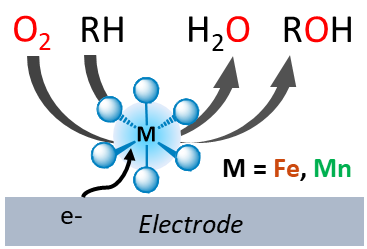
O2 activation
Developing sustainable and eco-friendly chemical processes using molecular O2 for oxidative transformation of organic molecules in place of the commonly used environmentally damaging and/or energy demanding processes is an important issue. In Nature, metallo-enzymes such as Cytochromes P450 are able to activate O2 to perform oxidation reactions in mild conditions. Taking inspiration from these processes by designing biomimetic model metal complexes, we develop new experimental and mechanistic approaches to examine and understand the mechanisms and key intermediates involved in O2 activation.
Electrifying organometallic catalysis, toward green electrosynthesis
We are combining homogeneous catalysis and electrochemistry in order to develop atom and energy efficient transformations, ranging from CO2 reduction to anodic C-H bond activations. A dual experimental and theoretical approach is developed to both rationalize experimental findings and to benchmark the theory used to model the catalytic systems. Overall, these studies seek to explore novel approaches towards electrifying homogeneous catalysts for energy storage applications.”
